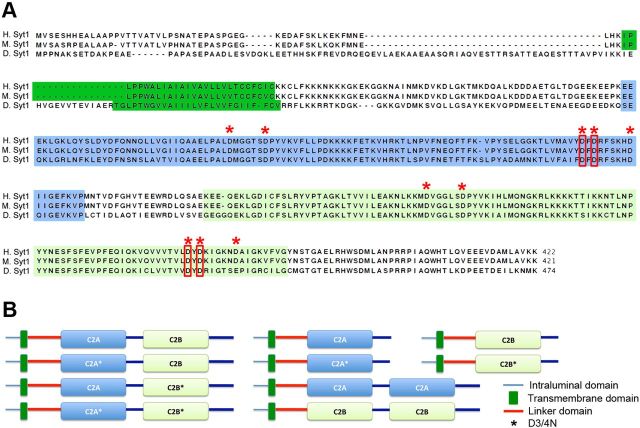
Figure 1.
Syt1 transgenic constructs and sequence similarity. A, Amino acid sequences of human, mouse, and Drosophila Syt1 are compared. A single transmembrane domain and two C2 domains, C2A and C2B, are indicated as dark green, blue, and light green blocks, respectively. The five aspartate residues (D) involved in binding Ca2+ ions in each C2 domain are indicated with red asterisks. The third and fourth of these five aspartate residues were mutated to asparagines (N) to disrupt Ca2+-binding ability of each C2 domain (red boxes). B, The design of transgenic UAS-Syt1 constructs used for the analysis is shown. Syt1 consists of a short intraluminal region (blue line), a single transmembrane domain (dark green box), a cytoplasmic linker (red), two C2 domains (C2A, blue; C2B, green) with a short linker between them, and a C-terminal tail. *C2 domains containing mutations (described in A) (red boxes).