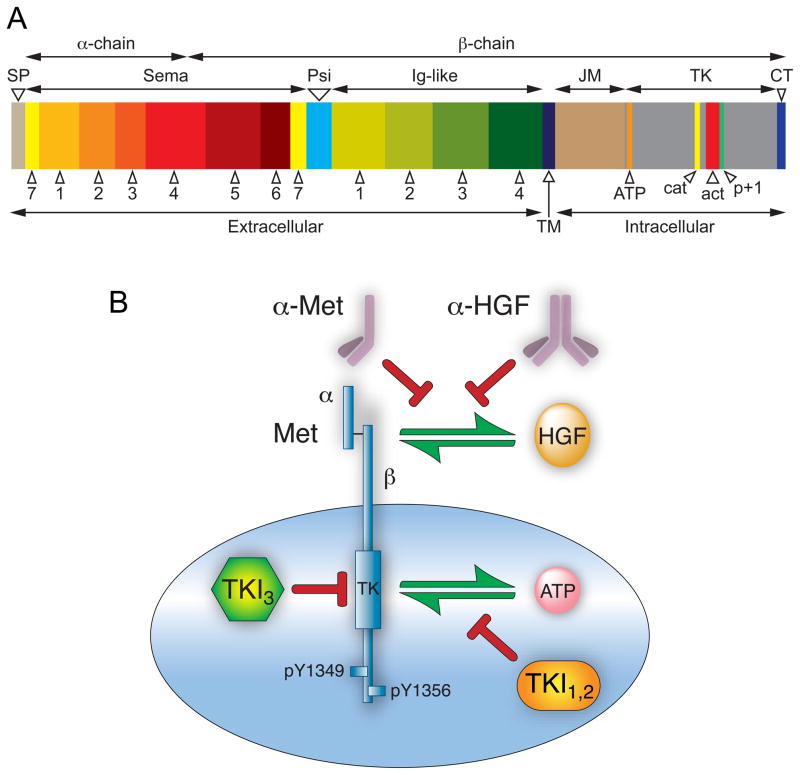
Figure 1. Met domain structure and routes to antagonize the HGF/Met pathway.
A. Schematic of Met domain structure; domain lengths are proportional to number of constituent amino acid residues. Mature Met is a disulfide-linked two chain heterodimer with an extracellular amino terminal α-chain (45 kDa) and a carboxyl terminal β-chain (145 kDa) containing extracellular, transmembrane and intracellular domains. The signal peptide (SP) is not present in mature protein. The extracellular domain contains a sema homology region (Sema) organized in 7 blades; a cysteine-rich region (Psi); and four immunoglobulin-like repeats (Ig-like). The intracellular domain contains juxtamembrane (JM), tyrosine kinase (TK) and carboxyl terminal (CT) domains. Within the TK domain are the ATP binding site (orange), catalytic loop (cat, yellow), activation loop (act, red) and p+1 loop (p+1, green). B. At least three routes of pathway intervention have been followed as selective Met anticancer drug development strategies: 1) fully human monoclonal antibodies that neutralize HGF by binding to one of its two Met binding sites; 2) monovalent (one-armed), monoclonal antibodies designed to bind to Met and inhibit HGF binding; and 3) tyrosine kinase inhibitors classified as type I, type II (TKI1,2) and type III (TKI3) as described in text.