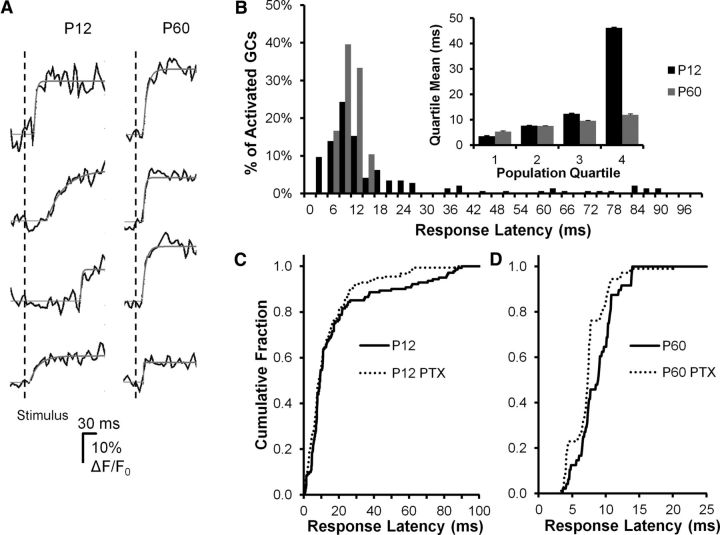
Figure 5.
DGC activation timing precision increases during postnatal development. A, Sample DGC calcium imaging traces from a P12 (left) and a P60 animal, demonstrating variability in response onset time after PP stimulation between groups. Onset time is estimated from exponential fits (solid lines, see Materials and Methods). The P12 response onsets are more variable, with frequent later events. PP stimulus time is indicated by the vertical line. B, Histogram plotting population percentage against response latency for P12 (black bars) and P60 (gray bars) animals. The late events are restricted to the P12 population, whereas P60 responses are limited to the first 15 ms after stimulation. These population distributions of response latency were significantly different (Kolmogorov–Smirnov test, p = 0.00068). Inset, Further illustration of the differences between groups by plotting the mean response latencies broken down by population quartiles. For the first three quartiles, there is very little difference between groups, whereas in the fourth population quartile, there is a fivefold longer average response latency in P12 DGCs. C, D, Sensitivity of response onset time to GABA antagonist (PTX, 50 μm) perfusion in P12 (C) and P60 (D) DGCs. Both populations exhibited PTX sensitivity in response latencies, but the nature of these effects differed. For P12 DGCs (C), PTX specifically blocked late activating responses (>20 ms latency), with little or no effect on early responses (0–15 ms), which constituted the majority of the population. A Kolmogorov–Smirnov test for overall differences between populations was not significant (p = 0.123); however, untreated cells had a slightly longer mean latency (p = 0.043, 10.1 vs 7.7 s, t test after log-transforming the data). For P60 DGCs (D), PTX shifted the entire curve to the left (Kolmogorov–Smirnov test p < 0.0001), consistent with a significant decrease in response latency. P12: N = 3 animals, 28 fields, 141 cells. P60: N = 3 animals, 22 fields, and 48 cells.