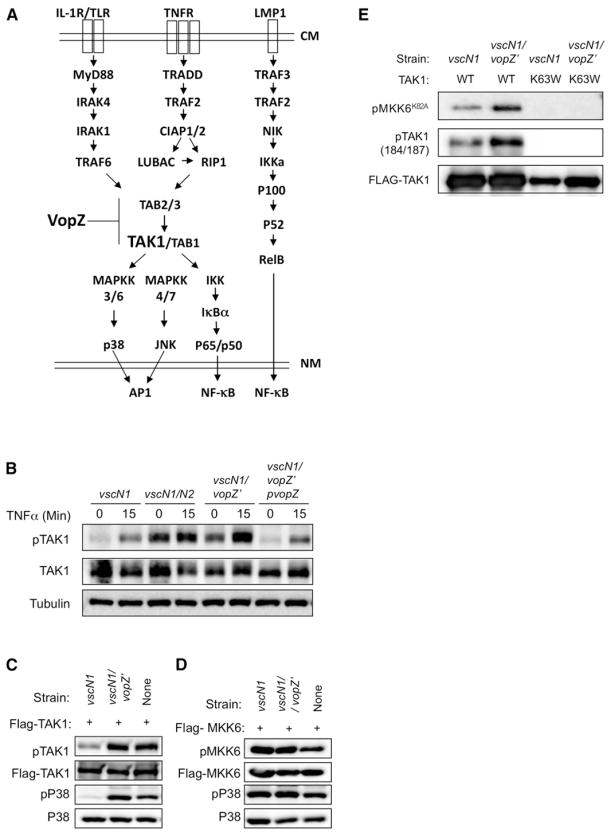
Figure 5. VopZ Inhibits TAK1 Activation.
(A) Schematic of signaling pathways leading to activation of MAPKs and canonical and noncanonical NF-κB, and possible site of VopZ action. CM, cell membrane; NM, nuclear membrane.
(B) HEK293 cells were infected with V. parahaemolyticus strains for 90 min and then stimulated by TNF-α (1 ng/ml). Activation of TAK1 was assessed using antibodies to total and T184/T187-phosphorylated TAK1.
(C and D) TAK1, but not MKK6, autophosphorylation and activity are inhibited by VopZ. FLAG-TAK1 or MKK6 was transiently expressed in HEK293 cells, which were then infected for 90 min with the indicated strains. Activation of TAK1, MKK6, and P38 was assessed using antibodies to total and phosphorylated forms of proteins.
(E) WT or K63W (kinase-deficient) TAK1 was immunopurified from transfected HEK293 cells that had been infected with the indicated strains for 90 min prior to harvest. TAK1 activity was monitored using a cold kinase assay, followed by detection of phosphorylated substrate (recombinant kinase-deficient MKK6-K82A) via immunoblotting. Phospho-and total TAK1 levels were also monitored by immunoblot. Western blots representative of three independent experiments are shown.