Abstract
It has been suggested for some time that circadian rhythm abnormalities underlie the development of multiple psychiatric disorders. However, it is unclear how disruptions in individual circadian genes might regulate mood and anxiety. Here we found that mice lacking functional mPeriod 1 (mPer1) or mPeriod 2 (mPer2) individually did not have consistent behavioral abnormalities in measures of anxiety-related behavior. However, mice deficient in both mPer1 and mPer2 had an increase in levels of anxiety-like behavior in multiple measures. Moreover, we found that mPer1 and mPer2 expression was reduced in the nucleus accumbens (NAc) after exposure to chronic social defeat stress, a paradigm that led to increased anxiety-related behavior. Following social defeat, chronic treatment with fluoxetine normalized Per gene expression towards wild-type levels. Knockdown of both mPer1 and mPer2 expression via RNA interference specifically in the NAc led to a similar increase in anxiety-like behavior as seen in the mutant animals. Taken together, these results implicate the Per genes in the NAc in response to stress and the development of anxiety.
Keywords: circadian rhythms, gene expression, mouse, psychiatric disease, stress, striatum
Introduction
Circadian rhythms are prominent in virtually every species on this planet and nearly all bodily and cognitive functions in humans follow a 24-h cycle (Ko & Takahashi, 2006). Several studies have found that disruptions in normal rhythms in the sleep/wake cycle can lead to a variety of health problems such as jet lag, shift workers’ syndrome, and even increase the risk for cancer and heart disease (Moser et al., 2006). It has become increasingly clear that circadian rhythms also contribute to differences in mood state, reward and motivation. Abnormal rhythms are strongly associated with psychiatric diseases like seasonal affective disorder, bipolar disorder, major depression, and drug addiction (McClung, 2007; Falcon & McClung, 2009). Moreover, many of the treatments used for these illnesses are known to modulate the circadian clock. Nonetheless, the exact role of rhythm disruptions in these diseases still remains elusive, as does the contribution of specific circadian genes in individual brain regions in the regulation of mood.
The circadian clock in mammals is regulated by a core transcriptional/translational loop that cycles over the course of 24 h (Ko & Takahashi, 2006). The suprachiasmatic nucleus (SCN) is the location of the central pacemaker in the brain; however, most circadian genes are widely expressed throughout the brain and in other organs. The central components are the circadian locomotor output cycles kaput (CLOCK) and brain and muscle ARNT-like protein 1 (BMAL1) proteins that dimerize and induce the expression of the Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes. The CRY and PER proteins can inhibit the activity of CLOCK and BMAL1, thus creating a negative feedback loop. Several other proteins, such as Rev-erbα and β, retinoid-related orphan receptor beta (RORβ), casein kinase 1 epsilon and glycogen synthase kinase 3 beta (GSK3β), are also involved in regulating the timing of these rhythms. It was shown previously that the three Period genes have differential functions in the regulation of circadian rhythms (Shearman et al., 2000; Bae et al., 2001). Mice with a mutation in either mPeriod 1 (mPer1) or mPeriod 2 (mPer2) have disrupted free-running rhythms with lower amplitude in constant darkness. However, most mice retain a significant period of approximately 22–23 h for several days in constant darkness before losing all rhythmicity (Bae et al., 2001). Animals with mutations in both mPer1 and mPer2 have a much more dramatic and immediate loss of rhythmicity in constant darkness, suggesting that PER1 and PER2 can compensate for one another to some extent when one protein is lost to help maintain circadian rhythms (Bae et al., 2001). In contrast, mice with a mutation in mPeriod 3 (mPer3) have surprisingly few disruptions in circadian locomotor activity. Indeed, mice with double mutations in mPer3 along with mPer1 or mPer2 do not have an increase in circadian rhythm disruption over the single mPer1 or mPer2 mutations alone, suggesting that this gene has a minimal role in core circadian clock function even though it displays rhythmic expression levels in the SCN (Shearman et al., 2000; Bae et al., 2001). However, recent studies suggest a more tissue-specific role for mPer3 in the circadian timekeeping of peripheral oscillators throughout the body, like the liver, lung, and esophagus, among others (Pendergast et al., 2012).
Several human genetic studies have identified single nucleotide polymorphisms and haplotypes in individual circadian genes that associate with various psychiatric disorders. For example, variations in Clock, Bmal1, GSK3β, Per3, RORβ and Rev-erbα have all been linked to a bipolar disorder diagnosis or various aspects of bipolar disorder (Benedetti et al., 2004; Nievergelt et al., 2006; Benedetti et al., 2007; Kishi et al., 2008; Kripke et al., 2009; McGrath et al., 2009). Moreover, polymorphisms in Per2 are associated with vulnerability to major depression and seasonal depression, as well as alcoholism (Spanagel et al., 2005; Partonen et al., 2007; Lavebratt et al., 2010), and levels of Clock, Per1 and Bmal1 mRNA are elevated in blood leukocytes of people with a history of depression (Gouin et al., 2010). Recently, single nucleotide polymorphisms in Bmal2 (a functional homologue to Bmal1) have been identified that associate with social phobia anxiety-related disorders (Sipila, 2010).
Work in animal models has found that mice with a mutation in the Clock gene (ClockΔ19) (King et al., 1997; Vitaterna et al., 2006) have a complete behavioral profile that is strikingly similar to human bipolar patients in the manic state, including hyperactivity, lowered levels of anxiety-like behavior or increased “risk-taking” behavior, lowered levels of depression-like behavior, and an increase in the reward value for a variety of stimuli (McClung et al., 2005; Roybal et al., 2007). A few studies have similarly begun to examine the influence of the Per genes on mood and reward-related behavior in mice (Abarca et al., 2002; Spanagel et al., 2005; Halbout et al., 2011). Here we wanted to examine the influence of the Per genes on anxiety-related behavior and start to understand the mechanisms by which these genes are involved in the response to stress.
Materials and methods
Mice
The homozygous mPer1ldc, mPer2ldc, and mPer1;mPer2ldc mice used in this study were generously provided by David Weaver and colleagues at UMass Medical School (Shearman et al., 2000; Bae et al., 2001) and bred and genotyped at UT Southwestern Medical Center at Dallas. All mutant mice were on a 129sv background. Individual wild-type (WT) littermates generated from heterozygote breeding were used as controls for all single gene knockouts and combinations of these WT animals from the single mutation crosses were compared with the double knockout animals (which were maintained as homozygotes for both mutations). No behavioral differences were found between WT animals from the mPer1 or mPer2 crosses (data not shown). C57BL/6J mice used in RNA interference were purchased from Jackson Laboratory and group housed for at least 1 week prior to stereotaxic surgery. Mice used in social defeat experiments are described in detail below. All animals were maintained in a 12:12 light/dark cycle (lights on at 07:00 h) with food and water freely available. Experimental mice used in behavioral analysis were adult males (8–12 weeks old) and behavioral tests of locomotor activity, anxiety, and depression-related behavior were conducted between ZT 3 and ZT 6 (social defeat experiments are detailed below). All procedures were approved by our Institutional Animal Care and Use Committee at UT Southwestern.
Behavioral tests
We utilized a battery of behavioral tests to phenotype mice working from the least to most stressful as previously characterized (Tarantino & Bucan, 2000; Paylor et al., 2006). Importantly, supporting research indicates that there is little to no effect on behavioral performance between inter-test intervals of 1 week compared with 1–2 days allowing for rapid and semi-high-throughput screening of behavior (Paylor et al., 2006). An inter-test interval of 1–2 days was adopted for this study in line with our previously published results (Roybal et al., 2007; Mukherjee et al., 2010).
Locomotor response to novelty
Mice were individually placed in automated locomotor activity chambers equipped with infrared photobeams (San Diego Instruments, San Diego, CA, USA) and measurements began immediately. The activity of the animal was continuously measured and the data were collected in 5-min blocks over a period of 2 h.
Elevated plus maze
Mice were placed in the center of an elevated plus maze (EPM) (arms are 30 × 5 cm, with 25-cm-high walls on the closed arms) under low light levels and their behavior was monitored for 5 min. The time spent on the closed and open arms, as well as the number of explorations of open and closed arms, were determined by video tracking software (Ethovision 3.0, Noldus, Leesburg, Virginia, USA). The apparatus was cleaned and allowed to dry between mice.
Dark/light test
The dark/light apparatus consisted of two-chambered boxes (25 × 26 cm for each side) (Med Associates, St Albans, Vermont, USA). One side was kept dark (room light entry limited) and the other side was brightly lit by a fluorescent bulb across the top. Mice were first placed in the dark side for 2 min, then the door between the compartments was opened and they were allowed to freely explore either the light or dark side for 10 min. Anxiety-like behavior was measured as the activity in the lit side during the final 10 min.
Open field test
Mice were placed in the periphery of a novel open field environment (44 × 44 cm, 30-cm-high walls) in a dimly lit room and allowed to explore for 5 min as described previously (Roybal et al., 2007). The animals were monitored from above by a video camera connected to a computer running video tracking software (Ethovision 3.0, Noldus) to determine the time, distance moved and number of entries into two areas: the periphery (5 cm from the walls) and center (14 × 14 cm). The open field arenas were wiped and allowed to dry between mice.
Social defeat and avoidance testing
Social defeat and avoidance testing were performed according to published protocols (Berton et al., 2006; Tsankova et al., 2006; Krishnan et al., 2007). Briefly, CD1 retired breeder mice (purchased from Jackson Laboratory) were screened for consistent attack latencies (<30 s on three consecutive screening sessions with a C57BL/6J intruder). C57BL/6J mice were purchased from Jackson Laboratory and group housed in a 12-h light/dark cycle with food and water ad libitum for at least 1 week prior to the defeat protocol in our facility. During each defeat episode, intruder mice were allowed to interact for 10 min with an unfamiliar aggressive CD1 mouse, during which they were attacked and displayed subordinate posturing. Non-defeated controls were housed in identical cages opposite each other and were rotated similarly. Immediately after the tenth defeat all mice were singly housed. Social defeat was always performed in the few hours before the onset of the dark phase (17:30–18:30 h). Social interaction tests were performed 24 h after the last defeat and then again after the last day of antidepressant treatment (fluoxetine pellets implanted s.c. for 28 days). On these days the time spent in the interaction zone during the first (target absent) and second (target present) trials was measured and the interaction ratio was calculated as 100 × (interaction time, target present)/(interaction time, target absent). Previous work has found that depression-related behavior persists for 28 days after the social defeat protocol and that antidepressant treatment completely reverses the social interaction deficit seen in susceptible animals (Berton et al., 2006; Tsankova et al., 2006). Animals were killed 24 h after the last fluoxetine or vehicle treatment and nucleus accumbens (NAc) dissections were taken by punch dissection between ZT 4 and ZT 6 as described previously (McClung & Nestler, 2003).
Quantitative polymerase chain reaction
The cDNA was mixed with buffer, primers, SYBR green, and hot-start Taq polymerase in a prepared master mix (Applied Biosystems, Foster City, CA, USA). The polymerase chain reactions followed by a dissociation reaction to determine the specificity of the amplified product were run on a Real-Time polymerase chain reaction machine (7300 Real-Time PCR machine, Applied Biosystems). The amount of gene expression was quantified using the ΔΔCt method as previously described (LaPlant et al., 2009). The following primer sets were used to measure Per expression: mPer1 forward, CTCTGTGCTGAAGCAAGACCG; mPer1 reverse, TCATCAGAGTGGCCAGGATCTT; mPer2 forward, GAGTGTGTGCAGCGGCTTAG; and mPer2 reverse, GTAGGGTGTCATGCGGAAGG.
Construction of mPer1;mPer2 small hairpin RNA
A small hairpin RNA (shRNA) was constructed against mPer1 and mPer2 mRNA by selecting a conserved 24-base sequence (5′-ATCCCTCCTGACAAGAGGATCTTC-3′) in the coding region. For the scrambled shRNA, a random sequence of 24 bases (5′-CGGAATTTAGTTACGGGGATCCAC-3′) that had no sequence similarities with any known genes/mRNA was used. An antisense sequence of the selected mRNA region followed by an miR23 loop of 10 nucleotides (CTTCCTGTCA) was added at the 5′ end of the above sequences. These shRNAs were designed as synthetic duplexes with overhang ends identical to those created by Sap I and Xba I restriction enzyme digestion. The annealed oligonucleotides were cloned into the adeno-associated virus (AAV) plasmid expressing enhanced green fluorescent protein (Stratagene, La Jolla, CA, USA). Plasmids were sent to the University of North Carolina Viral Vector Core for production (Chapel Hill, NC, USA).
Stereotaxic surgery
Stereotaxic surgery was performed as described in Mukherjee et al. (2010). Mice were anesthetized with a mixture of ketamine (50 mg/kg body weight) and xylazine (10 mg/kg body weight) in saline (0.9% NaCl). Bilateral stereotaxic injections of 1 µL of purified high-titer AAV encoding scrambled or AAV-mPer1;mPer2 shRNA was injected into the NAc (from bregma: angle 10°; AP, +1.5 mm; Lat, +1.5 mm; DV, −4.4 mm) using a 33-gauge hamilton syringe (Hamilton, Reno, NV, USA). The injection speed was 0.1 µL/min, and the needle was kept in place for an additional 5 min before it was slowly withdrawn. Mice recovered for 2 weeks in their home cage prior to behavioral testing to allow for full virus expression.
Immunohistochemical localization of adeno-associated virus expression
Mice were anesthetized with 50 mg/kg sodium pentobarbital in saline, and transcardially perfused with 4% paraformaldehyde in 1× phosphate-buffered saline (1 mM KH2PO4, 10 M Na2HPO4, 137 mM NaCl, 2.7 mM KCl, pH 7.4). The brains were allowed to post-fix in 4% paraformaldehyde for 24 h and then placed in 1× phosphate-buffered saline/30% glycerol sucrose protection for an additional 24 h before being stored in 1× phosphate-buffered saline/0.05% sodium azide. Brain sections (30 µm) were obtained with a microtome (Leica, Wetzlar, Germany, USA) and immunohistochemical staining against GFP (AbCam, Cambridge, MA, USA) was carried out using standard procedures. Secondary antibodies (anti-rabbit conjugated with Alexa 488) were purchased from Molecular Probes (Carlsbad, CA, USA). Brain sections were mounted using Vectashield (Vector Labs, Burlingame, CA, USA) with DAPI counterstaining and observed with an epifluorescence microscope with a 10× objective. Animals were excluded from our study if their infection spread was not localized to the NAc with spillover to adjacent areas or throughout the injection tract, or if there was a significant disproportionate amount of infection between both sides. Exclusion by these criteria accounted for approximately 10% of animals.
Statistical analysis
All data are expressed as mean ± SEM. Significance for two group comparisons in behavioral assays was determined by one-way ANOVA and post-hoc analysis. Behavioral results from the shRNA experiments and qPCR analysis were analyzed by Student’s t-test. In all experiments P<0.05 is considered significant.
Results
mPeriod 1 and mPeriod 2 are involved in regulating anxiety-related behavior
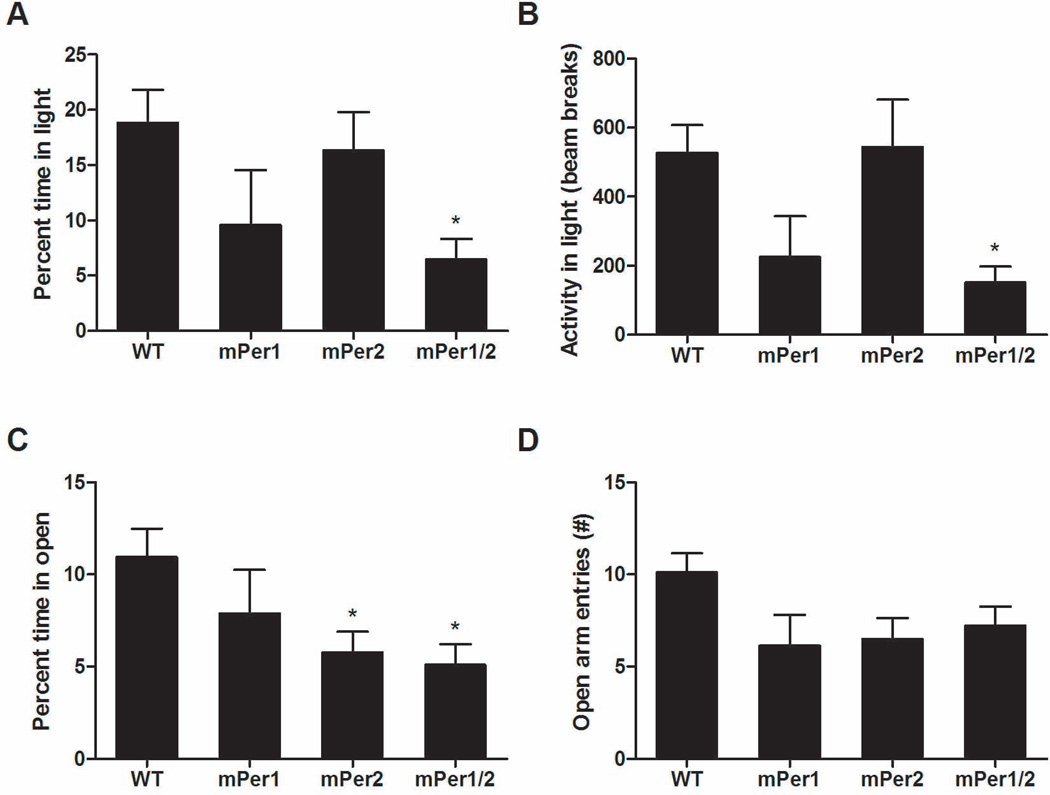
To determine if mice deficient in mPer1, mPer2, or both have any anxiety-related behavioral abnormalities, we utilized the EPM and dark/light tests. These tests have been validated extensively as a measure of anxiety-related behavior and for their sensitivity to anxiolytic and anxiogenic drugs (Belzung & Griebel, 2001). Compared with WT animals, we found a significant decrease in the percentage of time spent in the light side of a dark/light chamber in the mPer1;mPer2 double mutant mice (Fig. 1A) (one-way ANOVA, F3,51 = 2.771, P = 0.05; post-hoc Bonferroni test, P < 0.05). Likewise, in Fig. 1B, when we examined activity on the light side of the dark/light chamber there was a sizeable decrease in activity in mice lacking both mPer1 and mPer2 (one-way ANOVA, F3,51 = 3.579, P = 0.02; post-hoc Bonferroni test, P < 0.05). Importantly, when we examined total activity for the 10-min testing period, there was no difference between the genotypes (Supplementary Fig. 1A). Mice deficient in mPer2 alone were similar to WT animals in the dark/light test, whereas mice deficient in mPer1 showed a non-significant trend towards a decrease in the percent time and activity in the light. In the EPM, analysis of percent time spent in the open arms of the EPM was statistically significant between groups as measured by one-way ANOVA in Fig. 1C (F3,47 = 3.279, P = 0.029), whereas Bonferroni post-hoc tests revealed a significant decrease in time spent in the open arms for both mPer2 single and mPer1;mPer2 double mutant mice (P < 0.05). Analysis of the number of open arm entries nearly met significance by one-way ANOVA (Fig. 1D) (F3,47 = 2.692, P = 0.0568) with a trend towards a decrease in open arm entry number for mPer1 (6.125 ± 1.695), mPer2 (6.50 ± 1.142), and mPer1;mPer2 double mutants (7.222 ± 1.051) as compared with WT mice (10.10 ± 1.044). There were no differences between genotypes in the total crosses, percent time in the closed arm, or time in the center of the EPM, indicating no general change in activity (Supplementary Fig. 1B, D and F). Additional analysis in the open field test revealed a strong trend toward a decrease in the frequency of times that the mPer1;mPer2 mutant mice spent in the center of the field when analyzed by one-way ANOVA (WT mean, 4.9 ± 0.90; mPer1;mPer2 mean, 2.10 ±0.83; mPer1 mean, 2.88 ± 0.81; mPer2 mean, 4.8 ±0.99; F3,67=2.551, P=0.0629) with no change in total activity, in support of the overall conclusion that mPer1;mPer2 mutant mice displayed increased anxiety-like behavior. Importantly, as the single mPer1 and mPer2 deficient lines showed greater inconsistency in these measures, this suggests that each protein is likely able to compensate to some extent for the loss of the other.
Fig. 1.
mPer1;mPer2 mutant mice display increased anxiety-related behavior. (A) mPer1;mPer2 mutant mice spent less time in the lighted compartment of the dark/light box. *P < 0.05, one-way ANOVA followed by Bonferroni post-hoc test (n = 25, WT; n = 7, mPer1; n = 12, mPer2; n = 11, mPer1/2). (B) mPer1;mPer2 mutant mice had less locomotor activity in the light as measured by beam breaks in the lit compartment of a dark/light box compared with WT mice. *P < 0.05, one-way ANOVA followed by Bonferroni post-hoc test (n = 20, WT; n = 8, mPer1; n = 14, mPer2; n = 9, mPer1/2). (C) mPer2 and mPer1;mPer2 mutant mice spent less time in the open arms of the EPM. *P < 0.05, one-way ANOVA followed by Bonferroni post-hoc test (n = 25, WT; n = 7, mPer1; n = 12, mPer2; n = 11, mPer1/2). (D) Open arm entry frequency in the EPM (n = 20, WT; n = 8, mPer1; n = 14, mPer2; n = 9, mPer1/2).
Mutations in the Period genes do not alter the locomotor response to novelty
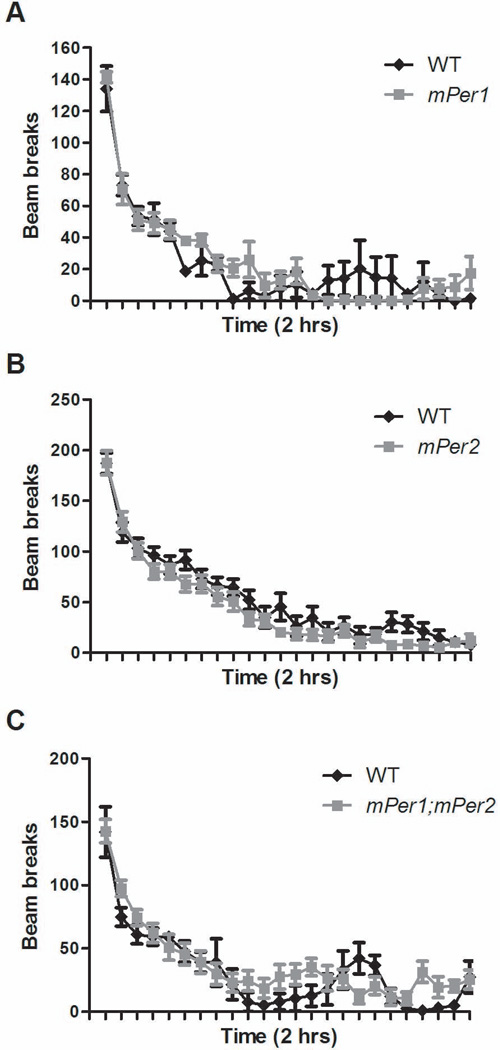
Mice with a mutation in the Clock gene are hyperactive in response to a novel environment (Roybal et al., 2007). Thus, we wanted to determine if mice with a mutation in either mPer1, mPer2, or mPer1;mPer2 had any differences in novelty-induced activity. Mice were placed in an unfamiliar chamber for 2 h and beam breaks were counted in 5-min bins. We found that loss of Period gene function did not lead to significant changes in total locomotor activity between all genotypes tested (F3,43 = 2.181, P = 0.1041) (Fig. 2). These results suggest that mPer1 and mPer2 are not involved in the regulation of the locomotor response to novelty. These results also confirm that the decreased exploratory behavior seen in the anxiety-related tests was not due to an overall decrease in locomotor activity.
Fig. 2.
Mice with mutations in the Per genes have a normal locomotor response to novelty. Locomotor activity of (A) mPer1 (n = 5), (B) mPer2 (n = 14), and (C) mPer1;mPer2 (n = 9) mutant mice was not significantly different from WT littermates (n = 20) by two-way ANOVA.
Chronic social defeat and antidepressant treatment alter Period gene expression in the nucleus accumbens
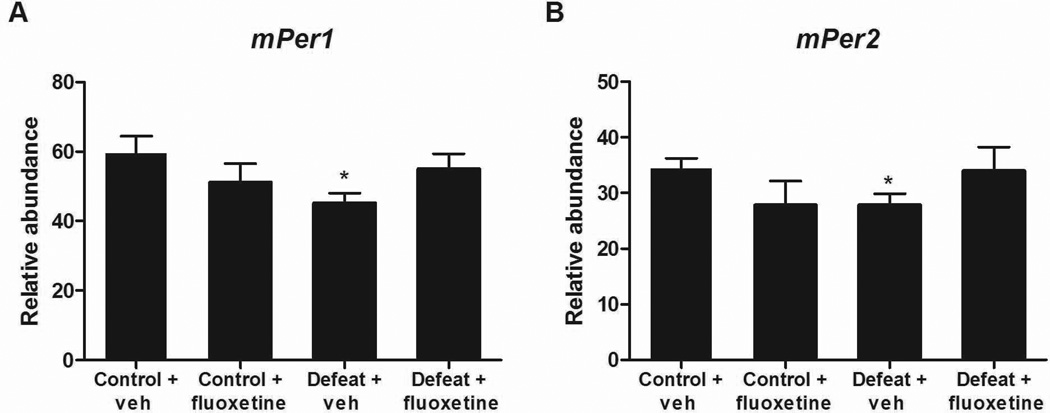
As mice with mutations in both Per genes have significant behavioral phenotypes in anxiety-related measures, we wanted to know if chronic stress or anxioloytic/antidepressant treatment would alter Per gene expression in the NAc. We decided to employ a social defeat paradigm in which mice are subjected to attacks by an aggressive mouse once per day for 10 days. They are also housed in an environment where they can see and smell the aggressor at all times during the 10-day protocol. Previous studies have found that, in the majority of mice, this paradigm leads to a profound increase in social avoidance behavior and anxiety-related behavior that is extremely long lasting, but can be reversed with chronic antidepressant treatment (Berton et al., 2006; Tsankova et al., 2006). Indeed, with this experiment, we found a highly significant decrease in social interaction following defeat that was rescued by 28 days of fluoxetine treatment (Supplementary Fig. 2). We chose to examine changes in expression in the NAc, which is highly involved in both depression and anxiety-related behavior because previous studies have found that social defeat leads to specific gene expression changes in this region (Nestler & Carlezon, 2006; Krishnan et al., 2007). Furthermore, Per gene expression is strong in striatal regions, and can be altered in these regions by other behavioral paradigms such as those that employ chronic exposure to drugs of abuse (Nikaido et al., 2001; Iijima et al., 2002). When we measured Per gene expression we found that mice that had been exposed to social defeat stress for 10 days had a significant decrease in both mPer1 (T13 = 2.387, P = 0.0329) and mPer2 (T13 = 2.33, P = 0.0366) expression (Fig. 3). Furthermore, mice that received fluoxetine treatment following defeat had levels of both mPer1 and mPer2 that were not different from control mice, suggesting that antidepressant treatment normalizes Per gene expression (Fig. 3). Interestingly, control mice that did not experience the social defeat but did have chronic fluoxetine treatment had no significant changes in mPer1 or mPer2 (Fig. 3). These results demonstrate that chronic stress leads to a long-lasting decrease in Per gene expression in the NAc that is partially reversed by antidepressant treatment.
Fig. 3.
Chronic stress leads to a decrease in mPer1 and mPer2 expression in the NAc. WT mice were subjected to 10 days of social defeat stress followed by 28 days of vehicle (veh) (n = 8) or fluoxetine (n = 6) treatment (pellets implanted s.c.). Control mice were only subjected to the 28 days of vehicle (n = 7) or fluoxetine pellets (n = 6), but no stress. mPer1 and mPer2 mRNA expression was significantly decreased in the NAc following chronic stress (*p<0.05) (A and B). Fluoxetine treatment following social defeat stress resulted in expression levels that were not different from control animals.
Knockdown of mPeriod 1 and mPeriod 2 in the nucleus accumbens contributes to anxiety-related behavior
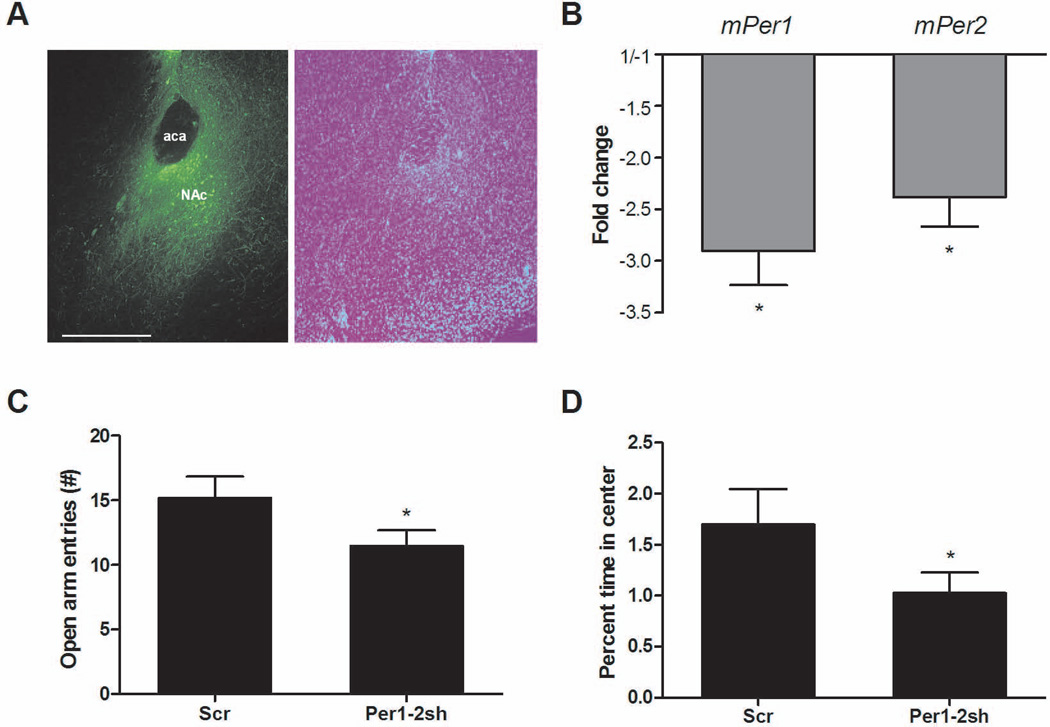
To determine if a knockdown of mPer1 and mPer2 gene expression specifically in the NAc would be sufficient to increase anxiety, we employed an AAV virus with a short hairpin RNA that targeted a common sequence in both mPer1 and mPer2. This leads to a decrease in the expression of both genes via RNA interference mechanisms. In mouse embryonic fibroblast cells, this AAV-shRNA led to large decreases in mPer1 and mPer2 expression (7.2-fold and 13.3-fold, respectively, data not shown) when compared with expression of an AAV-scrambled control shRNA that does not match the sequence of any known gene. Infection with the AAV-mPer1/mPer2 shRNA virus in the NAc of intact mice led to a more modest threefold decrease in mPer1 (T4 = 4.959, P = 0.0077) and a 2.4-fold decrease in mPer2 (T5 = 3.704, P = 0.0139) compared with controls (Fig. 4B). To ensure that this decrease was specific to mPer1 and mPer2, we measured levels of mPer3 and found no change in expression (data not shown). After waiting 2 weeks for full viral expression in the NAc, we subjected AAV-mPer1/mPer2 shRNA-infected mice and mice expressing the AAV-scrambled control shRNA to the EPM and open field. Even with this modest decrease in mPer1 and mPer2 expression in the NAc, we saw significant differences in anxiety-related behavior. In the EPM, there was a significant decrease in the number of open arm entries in the mPer1/mPer2 shRNA-infected mice (t19 =1.845, P = 0.04) (Fig. 4C). Total crosses, closed arm entry frequency and center time were similar between groups (Supplementary Fig. 1C, E and G). In addition, there was a significant decrease in percent time spent in the center of an open field in the mPer1/mPer2 shRNA-infected mice (t19 = 1.708, P = 0.05) (Fig. 4D). These data suggest that mPer1 and mPer2 expression in the NAc is important in the regulation of anxiety-related behavior.
Fig. 4.
Knockdown of mPer1 and mPer2 in the NAc increases anxiety. Mice were infused with the AAV-mPer1;mPer2 shRNA (sh) or AAV-scrambled control shRNA (Scr) into the NAc and behavior was measured 2 weeks later. (A) Representative image showing viral expression in the NAc and DAPI staining of the same slice. Scale bar, 500 µm. (B) AAV-mPer1;mPer2 infusion leads to a significant knockdown of mPer1 and mPer2 expression in the NAc (n = 6, Scr-infected mice; n = 5–6 mPer1;mPer2 shRNA-infected mice with two biological replicates of each). (C)AAV-mPer1;mPer2 shRNA NAc-infected mice (n = 10) had fewer open arm entries in the EPM than controls (n = 11) (P < 0.05, t-test). (D) AAV-mPer1;mPer2 shRNA NAc-infected mice spent less time in the center of the open field (*P<0.05, t-test).
Discussion
Our results show that the Per genes are involved in the regulation of anxiety-related behavior. Mice lacking both mPer1 and mPer2 have a robust increase in anxiety-related behavior, whereas the single gene mutants display inconsistent results across tests with mice lacking a functional mPer1 or mPer2 gene alone having greater anxiety-like behavior in a more restricted subset of measures. A recent study found that mPer1 mutant mice show enhanced alcohol consumption following social defeat stress relative to WT mice (Dong et al., 2011). Moreover, an SNP in the hPer1 promoter that leads to lowered cortisol-induced Per1 transcription was associated with psychosocial adversity and drinking in adolescents (Dong et al., 2011). In line with our results, these results suggest that lowered Per gene expression is associated with a heightened and anxiogenic response to stress.
It is interesting that the mPer1;mPer2 mutant mice have an increase in anxiety-related behavior, whereas mice with a mutation in the Clock gene have a decrease in anxiety-related behavior (Roybal et al., 2007). The Clock mutant mice are also hyperactive in response to novelty, whereas the Per gene mutants are normal in these measures. These results show that disruption in any of the core circadian genes does not result in a common behavioral profile. CLOCK and PER proteins have opposing activity in the circadian loop with PER proteins acting as inhibitors of CLOCK. Therefore, perhaps an opposing behavioral phenotype in these measures is not surprising if it is dependent upon CLOCK activity. Interestingly, SCN lesions lead to an antidepressant effect in measures of behavioral despair, but no change in anxiety-related behavior (Engelmann et al., 1998; Tataroglu et al., 2004; Tuma et al., 2005). This suggests that the anxiogenic effects seen in the mPer1;mPer2 mutant mice are due to a lack of Per gene function in other brain regions outside the SCN.
It is recognized that anxiety and depression occur more frequently in females compared with males in the human population (Palanza, 2001). A few laboratories have investigated gender-specific differences in stress responses and anxiety-related behaviors with mixed results. In one set of studies, female rats displayed heightened anxiety-like behavior in the Vogel conflict test, whereas in the EPM male rodents were the more anxious of the two (Johnston & File, 1991). In a more recent study examining NPY knockout mice, although the genetic manipulation increased anxiety-like responses in both sexes, the effect was more pronounced in males compared with females (Karl et al., 2008). In addition, working with female rodents requires consideration of the estrous cycle, adding an additional experimental variable with indices of anxiety-related behavior affected by cycle phase (Mora et al., 1996). In the present investigation, male mice were exclusively utilized in the behavioral assays for a number of reasons including those outlined above. We used tests of anxiety-related behavior that have been well-validated in male rodents. The chronic social defeat stress paradigm, for instance, is applicable only for male rodents with valid defeat stress models lacking for female rodents. Male and female rodents respond very differently to similar stressors, thus the same tests may not be suitable for the two populations. For instance, although male rodents have greater levels of anxiety-like behavior after exposure to a single restraint stress but adapt upon repeated exposure, female rodents display the opposite phenotype (Kennett et al., 1986). It is recognized that these studies will need to be extended to female mice in the future to understand the biological mechanisms underlying gender differences in anxiety-related behavior. Intriguingly, previous studies have implicated the circadian proteins CLOCK and PER2 in regulation of the female reproduction and estrous cycle (Pilorz & Steinlechner, 2008; Shimizu et al., 2011). Furthermore, it is known that sex hormones affect circadian rhythms (Iwahana et al., 2008), thus there may be some dynamic feedback between the two systems in the brain.
We decided to examine changes in expression in the NAc, which is highly involved in regulating behaviors in response to emotional stimuli because previous studies have found that social defeat leads to specific gene expression changes in this region (Nestler & Carlezon, 2006; Krishnan et al., 2007). Furthermore, Per gene expression is strong in striatal regions, and can be altered in these regions by other behavioral paradigms such as those that employ chronic exposure to drugs of abuse (Nikaido et al., 2001; Iijima et al., 2002). There is a growing body of literature implicating the NAc in the response to stress and in particular the development of anxiety (Barrot et al., 2002; Krishnan & Nestler, 2008; Chen et al., 2012). Importantly, the NAc is situated to integrate information from a large number of brain structures involved in processing stress and anxiety-related responses, including the prefrontal cortex, hypothalamus, hippocampus, and basolateral amygdala. Moreover, most measures of anxiety-like behavior in rodents evoke the conflict between trepidation and the desire to explore a novel environment, which is highly NAc dependent. A recent fMRI study conducted in human subjects demonstrated that the NAc is activated and inactivated during active and passive avoidance, respectively, and the degree of activation/deactivation correlates with individual levels of anxiety (Levita et al., 2012). These data demonstrate that the NAc is important for both positively and negatively influenced motivated behavior. In the present investigation, chronic social defeat stress led to a decrease in Per gene expression in the NAc at 28 days after the last defeat. Moreover, treatment with fluoxetine resulted in levels of both Per genes that were not different from control mice. Similar results were seen following treatment with a tricyclic antidepressant, imipramine (data not shown). Interestingly, a recent study (Koresh et al., 2012) found that expression of mPer1 and mPer2 was elevated in the hippocampus, frontal cortex and SCN at 8 days after exposure to predator scent stress in animals with an “extreme” (i.e. PTSD-like) behavioral response. Immediate treatment with agomelatine reversed these changes. Conversely, PER2 levels were decreased in the SCN but not hippocampus or prefrontal cortex in a time-specific manner in a study that employed chronic restraint as the stressor (Kinoshita et al., 2012). Night-time administration of the mood-stabilizer lithium normalized the stress-induced change in PER2 (Kinoshita et al., 2012). This suggests that there are widespread disruptions in Per gene expression throughout the brain following stress, and whether the changes are upwards or downwards might be specific to the region of the brain, type of stressor, amount of time following stress, and the time of day at which changes are measured.
Per gene expression appears to be particularly important in the NAc, as we find that local knockdown in this region is sufficient to increase anxiety-related behavior. Indeed, a relatively modest two- to threefold decrease in expression level for mPer1 and mPer2 was sufficient to produce a significant behavioral effect. It has been reported that the levels of the mPer1 transcript in the striatum fluctuate naturally by approximately threefold in a 24-h period (Uz et al., 2003). Research indicates that both the abundance and circadian oscillation pattern of the PERs are critically important for the circadian clock mechanism both in vitro and in vivo (Lee et al., 2011; Chen et al., 2012), thus it is perhaps not surprising that such a modest change could result in a significant outcome. The interpretation of constitutive shRNA knockdown data for a circadian rhythm-related gene is complicated because it cannot be said whether the effects are due to a change in transcript levels, rhythms or some combination of the two.
High rates of comorbidity between substance use and anxiety disorders have been well documented (Compton, 2007). Thus, the anxiogenic phenotype of the mPer1;mPer2 mutants and the mPer1;mPer2 shRNA knockdown in the NAc is particularly interesting in light of the reward-related phenotypes that have previously been reported in these mice. Interestingly, in 75% of cases in which anxiety disorders are comorbid with substance use disorders, the anxiety-related diagnosis pre-dates the substance use problem (Kushner et al., 2008). Although these data do not point to causality, they may suggest directionality with anxiety recognized as a risk factor for substance use disorders. It has been shown that mPer1 mutants fail to sensitize to the locomotor stimulating effects of cocaine or develop expression of cocaine conditioned place preference (Abarca et al., 2002), although more recent data show that these deficits do not extend to operant self-administration and reinstatement of cocaine-seeking behavior (Halbout et al., 2011). Conversely, mPer2 mutants show a hypersensitized response to cocaine with normal expression of conditioned place preference (Abarca et al., 2002). These studies also extend to other drugs of abuse. The mPer2 mutants display enhanced voluntary consumption of alcohol (Spanagel et al., 2005), whereas mPer1 expression is involved in morphine reward via extracellular signal-related kinase signaling mechanisms (Liu et al., 2007). Taken together, these data point to the involvement of the circadian PER proteins in reward-related behavior in addition to this newly defined role in anxiety-related behavior. As drug abuse and dependence are highly sensitive to stress and mood state, in the future it will be interesting to determine how these phenotypes interact in the mPer1 and mPer2 mutant animals.
In summary, mPer1 and mPer2 in the NAc appear to be centrally involved in the response to stress and development of anxiety-related behavior. Our results also suggest that an increase in these proteins in the NAc could be beneficial in the reversal of anxiety-related behavior following chronic stress. Thus, the Per genes represent new potential therapeutic targets for the treatment of anxiety disorders.
Supplementary Material
Acknowledgements
We thank Dr Will Renthal for assistance with tissue preparation and Ami Graham for assistance with the behavioral assays. We thank Dr Eric Nestler for assistance with the social defeat tissue collection and useful discussions. We also thank Dr Jude Samulsky and the UNC Gene Therapy Vector Core for their work on the viral constructs. This study was funded by the NIMH (MH082876).
Abbreviations
- AAV
adeno-associated virus
- BMAL1
brain and muscle ARNT-like protein 1
- CLOCK
circadian locomotor output cycles kaput
- Cry
Cryptochrome
- EPM
elevated plus maze
- GSK3β
glycogen synthase kinase 3 beta
- mPer1
mPeriod 1
- mPer2
mPeriod 2
- mPer3
mPeriod 3
- NAc
nucleus accumbens
- Per
Period
- RORβ
retinoid-related orphan receptor beta
- SCN
suprachiasmatic nucleus
- shRNA
small hairpin RNA
- WT
wild-type
Footnotes
The authors declare no conflicts of interest with this article.
References
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci U.S.A. 2002;99:9026–9030. doi: 10.1073/pnas.142039099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae K, Jin X, Maywood ES, Hastings MH, Reppert SM, Weaver DR. Differential functions of mPer1, mPer2, and mPer3 in the SCN circadian clock. Neuron. 2001;30:525–536. doi: 10.1016/s0896-6273(01)00302-6. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci U.S.A. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Bernasconi A, Lorenzi C, Pontiggia A, Serretti A, Colombo C, Smeraldi E. A single nucleotide polymorphism in glycogen synthase kinase 3-beta promoter gene influences onset of illness in patients affected by bipolar disorder. Neurosci Lett. 2004;355:37–40. doi: 10.1016/j.neulet.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, Colombo C, Smeraldi E. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Chen YW, Rada PV, Bützler BP, Leibowitz SF, Hoebel BG. Corticotropin-releasing factor in the nucleus accumbens shell induces swim depression, anxiety, and anhedonia along with changes in local dopamine/acetylcholine balance. Neuroscience. 2012;206:155–166. doi: 10.1016/j.neuroscience.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States. Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Dong L, Bilbao A, Laucht M, Henriksson R, Yakovleva T, Ridinger M, Desrivieres S, Clarke TK, Lourdusamy A, Smolka MN, Cichon S, Blomeyer D, Treutlein J, Perreau-Lenz S, Witt S, Leonardi-Essmann F, Wodarz N, Zill P, Soyka M, Albrecht U, Rietschel M, Lathrop M, Bakalkin G, Spanagel R, Schumann G. Effects of the circadian rhythm gene period 1 (per1) on psychosocial stress-induced alcohol drinking. Am J Psychiatry. 2011;168:1090–1098. doi: 10.1176/appi.ajp.2011.10111579. [DOI] [PubMed] [Google Scholar]
- Engelmann M, Ebner K, Landgraf R, Wotjak CT. Swim stress triggers the release of vasopressin within the suprachiasmatic nucleus of male rats. Brain Res. 1998;792:343–347. doi: 10.1016/s0006-8993(98)00243-1. [DOI] [PubMed] [Google Scholar]
- Falcon E, McClung CA. A role for the circadian genes in drug addiction. Neuropharmacology. 2009;56(Suppl 1):91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouin JP, Connors J, Kiecolt-Glaser JK, Glaser R, Malarkey WB, Atkinson C, Beversdorf D, Quan N. Altered expression of circadian rhythm genes among individuals with a history of depression. J Affect Disord. 2010;126:161–166. doi: 10.1016/j.jad.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbout B, Perreau-Lenz S, Dixon CI, Stephens DN, Spanagel R. Per1Brdm1 mice self-administer cocaine and reinstate cocaine-seeking behaviour following extinction. Behav Pharmacol. 2011;22:76–80. doi: 10.1097/FBP.0b013e328341e9ca. [DOI] [PubMed] [Google Scholar]
- Iijima M, Nikaido T, Akiyama M, Moriya T, Shibata S. Methamphetamine-induced, suprachiasmatic nucleus-independent circadian rhythms of activity and mPer gene expression in the striatum of the mouse. Eur J Neurosci. 2002;16:921–929. doi: 10.1046/j.1460-9568.2002.02140.x. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Hormones and Behavior. 2008;53:422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AL, File SE. Sex differences in animal tests of anxiety. Physiology & Behavior. 1991;49:245–250. doi: 10.1016/0031-9384(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Karl T, Duffy L, Herzog H. Behavioural profile of a new mouse model for NPY deficiency. European Journal of Neuroscience. 2008;28:173–180. doi: 10.1111/j.1460-9568.2008.06306.x. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Research. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TD, Vitaterna MH, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C, Miyazaki K, Ishida N. Chronic stress affects PERIOD2 expression through glycogen synthase kinase-3β phosphorylation in the central clock. NeuroReport. 2012;23:98–102. doi: 10.1097/WNR.0b013e32834e7ec2. [DOI] [PubMed] [Google Scholar]
- Kishi T, Kitajima T, Ikeda M, Yamanouchi Y, Kinoshita Y, Kawashima K, Okochi T, Ozaki N, Iwata N. Association analysis of nuclear receptor Rev-erb alpha gene (NR1D1) with mood disorders in the Japanese population. Neurosci Res. 2008;62:211–215. doi: 10.1016/j.neures.2008.08.008. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–R277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Koresh O, Kozlovsky N, Kaplan Z, Zohar J, Matar MA, Cohen H. The long-term abnormalities in circadian expression of Period 1 and Period 2 genes in response to stress is normalized by agomelatine administered immediately after exposure. European neuropsychopharmacology. 2012;22:205–221. doi: 10.1016/j.euroneuro.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Nievergelt CM, Joo E, Shekhtman T, Kelsoe JR. Circadian polymorphisms associated with affective disorders. J Circadian Rhythms. 2009;7:2. doi: 10.1186/1740-3391-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894–902. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner MG, Krueger R, Frye B, Peterson J. Epidemiological perspectives on co-occurring anxiety disorder and substance use disorder. In: Stewart SH, Conrod PJ, editors. USA: Springer; 2008. pp. 3–17. [Google Scholar]
- LaPlant Q, Chakravarty S, Vialou V, Mukherjee S, Koo JW, Kalahasti G, Bradbury KR, Taylor SV, Maze I, Kumar A, Graham A, Birnbaum SG, Krishnan V, Truong HT, Neve RL, Nestler EJ, Russo SJ. Role of nuclear factor kappaB in ovarian hormone-mediated stress hypersensitivity in female mice. Biol Psychiatry. 2009;65:874–880. doi: 10.1016/j.biopsych.2009.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C, Sjoholm LK, Partonen T, Schalling M, Forsell Y. PER2 variation is associated with depression vulnerability. American journal of medical genetics. Part B, Neuropsychiatric genetics. 2010;153B:570–581. doi: 10.1002/ajmg.b.31021. [DOI] [PubMed] [Google Scholar]
- Lee Y, Chen R, Lee H-M, Lee C. Stoichiometric relationship among clock proteins determines robustness of circadian rhythms. Journal of Biological Chemistry. 2011;286:7033–7042. doi: 10.1074/jbc.M110.207217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levita L, Hoskin R, Champi S. Avoidance of harm and anxiety: A role for the nucleus accumbens. NeuroImage. 2012;62:189–198. doi: 10.1016/j.neuroimage.2012.04.059. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang Y, Jiang Z, Wan C, Zhou W, Wang Z. The extracellular signal-regulated kinase signaling pathway is involved in the modulation of morphine-induced reward by mPer1. Neuroscience. 2007;146:265–271. doi: 10.1016/j.neuroscience.2007.01.009. [DOI] [PubMed] [Google Scholar]
- McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci U.S.A. 2005;102:9377–9381. doi: 10.1073/pnas.0503584102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath CL, Glatt SJ, Sklar P, Le-Niculescu H, Kuczenski R, Doyle AE, Biederman J, Mick E, Faraone SV, Niculescu AB, Tsuang MT. Evidence for genetic association of RORB with bipolar disorder. BMC Psychiatry. 2009;9:70. doi: 10.1186/1471-244X-9-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora S, Dussaubat N, Díaz-Véliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- Moser M, Penter R, Fruehwirth M, Kenner T. Why life oscillates - biological rhythms and health. Conf Proc IEEE Eng Med Biol Soc. 2006;1:424–428. doi: 10.1109/IEMBS.2006.259562. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao J-L, Kumar J, Chakravarty S, Asaithamby A, Graham A, Gordon E, Enwright JF, III, DiLeone RJ, Birnbaum SG, Cooper DC, McClung CA. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Schork NJ, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Palanza P. Animal models of anxiety and depression: how are females different? Neuroscience & Biobehavioral Reviews. 2001;25:219–233. doi: 10.1016/s0149-7634(01)00010-0. [DOI] [PubMed] [Google Scholar]
- Partonen T, Treutlein J, Alpman A, Frank J, Johansson C, Depner M, Aron L, Rietschel M, Wellek S, Soronen P, Paunio T, Koch A, Chen P, Lathrop M, Adolfsson R, Persson ML, Kasper S, Schalling M, Peltonen L, Schumann G. Three circadian clock genes Per2, Arntl, and Npas2 contribute to winter depression. Ann Med. 2007;39:229–238. doi: 10.1080/07853890701278795. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: Effect of test interval. Physiology & Behavior. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Niswender KD, Yamazaki S. Tissue-specific function of period3 in circadian rhythmicity. PLoS One. 2012;7:e30254. doi: 10.1371/journal.pone.0030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilorz V, Steinlechner S. Low reproductive success in Per1 and Per2 mutant mouse females due to accelerated ageing? Reproduction. 2008;135:559–568. doi: 10.1530/REP-07-0434. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U.S.A. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearman LP, Jin X, Lee C, Reppert SM, Weaver DR. Targeted disruption of the mPer3 gene: subtle effects on circadian clock function. Mol Cell Biol. 2000;20:6269–6275. doi: 10.1128/mcb.20.17.6269-6275.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Hirai Y, Murayama C, Miyamoto A, Miyazaki H, Miyazaki K. Circadian Clock genes Per2 and clock regulate steroid production, cell proliferation, and luteinizing hormone receptor transcription in ovarian granulosa cells. Biochemical and Biophysical Research Communications. 2011;412:132–135. doi: 10.1016/j.bbrc.2011.07.058. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med. 2005;11:35–42. doi: 10.1038/nm1163. [DOI] [PubMed] [Google Scholar]
- Tarantino L, Bucan M. Dissection of behavior and psychiatric disorders using the mouse as a model. Human Molecular Genetics. 2000;9:953–965. doi: 10.1093/hmg/9.6.953. [DOI] [PubMed] [Google Scholar]
- Tataroglu O, Aksoy A, Yilmaz A, Canbeyli R. Effect of lesioning the suprachiasmatic nuclei on behavioral despair in rats. Brain Res. 2004;1001:118–124. doi: 10.1016/j.brainres.2003.11.063. [DOI] [PubMed] [Google Scholar]
- Tsankova NM, Berton O, Renthal W, Kumar A, Neve RL, Nestler EJ. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat Neurosci. 2006;9:519–525. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- Tuma J, Strubbe JH, Mocaer E, Koolhaas JM. Anxiolytic-like action of the antidepressant agomelatine (S 20098) after a social defeat requires the integrity of the SCN. Eur Neuropsychopharmacol. 2005;15:545–555. doi: 10.1016/j.euroneuro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- Vitaterna MH, Ko CH, Chang AM, Buhr ED, Fruechte EM, Schook A, Antoch MP, Turek FW, Takahashi JS. The mouse Clock mutation reduces circadian pacemaker amplitude and enhances efficacy of resetting stimuli and phase-response curve amplitude. Proc Natl Acad Sci U.S.A. 2006;103:9327–9332. doi: 10.1073/pnas.0603601103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.