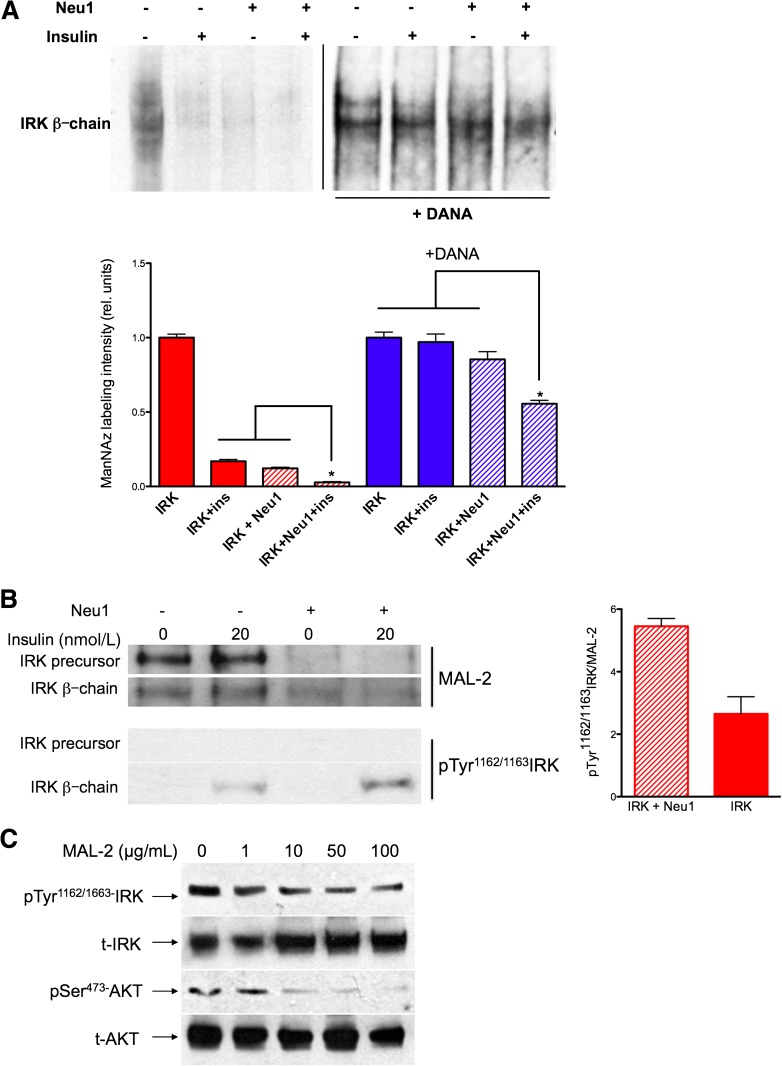
FIG. 3.
Insulin-dependent desialylation of IRK by Neu1. A: ManNAz labeling of sialic acid in the glycan chains of the β-subunit of IRK expressed in HEK cells alone or coexpressed with Neu1 and CathA and pretreated or not with 1 mmol/L DANA for 1 h. After 10 min of incubation with 20 nmol/L insulin, cells were solubilized with 1% octylglucoside and treated with biotin phosphane, and IRK was purified using affinity chromatography and analyzed by blotting with streptavidin-HRP. Bar graph shows quantification (mean values and SD) of signal intensities using ImageQuant software. In each experiment, the values were normalized to the intensities of IRK staining without Neu1 and insulin. The combined effect of Neu1 overexpression and insulin on ManNAz labeling was significantly (*P < 0.05) higher than that of each condition separately. B: IRK samples obtained as described above were studied by MAL-2 lectin blot (top) or Western blotting with anti-pTyr1162/1163 IRK antibodies. Equal loading of the IRK was verified with antibodies against IRK (not shown). Bar graph shows quantification (mean values and SD) of signal intensities for the β-subunit of IRK using ImageQuant software. C: MAL-2 blocks insulin-induced activation of IRK and AKT in a dose-dependent manner. HEK cells coexpressing CBP-tagged IRK and Neu1/CathA were treated with unlabeled MAL-2 lectin in a concentration of 0, 1, 10, 50, and 100 μg/mL of cell medium 30 min before induction with 20 nmol/L insulin for 10 min. Cells were solubilized in 50 mmol/L HEPES, pH 7.4, containing 0.1 mmol/L EDTA, 150 mmol/L sodium chloride, 2 mmol/L phenylmethylsulfonyl fluoride, 1% NP40, 2.5% sodium deoxycholate, and full protease and phosphatase inhibitor cocktail and subjected to immunoprecipitation using anti-IRK antibodies and Western blotting. Blots were stained with anti-pSer473 AKT and anti-AKT, anti-IRK β-chain, and anti-pTyr1162/1163 IRK antibodies as indicated.