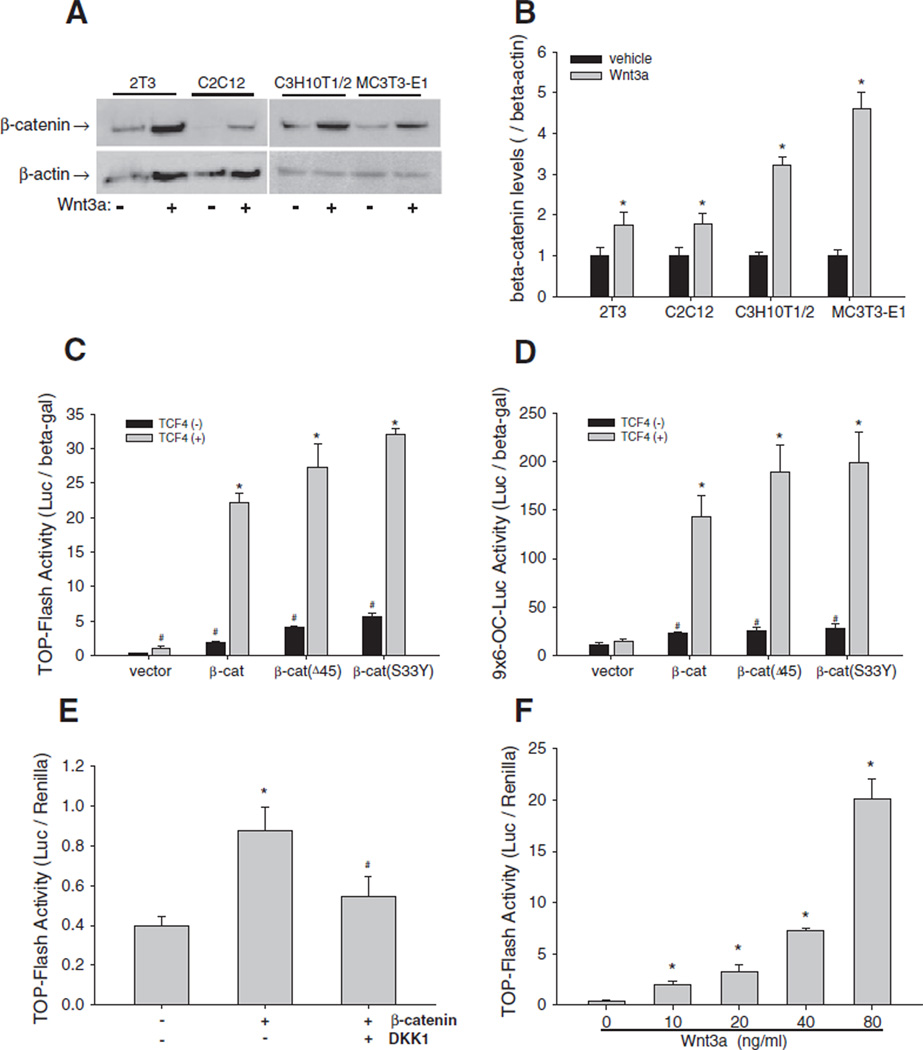
Fig. 1.
Wnt/β-catenin signaling in osteoblasts. (A and B) Effects of Wnt3a on β-catenin expression. 2T3, C2C12, C3H10T1/2, and MC3T3-E1 cells were treated with Wnt3a at 40 ng/mL for 24 h. β-catenin in cell lysates was detected by Western blot using an anti-β-catenin antibody. β-actin was used as control (A). The relative β-catenin levels were quantitated using Quantity One gel imaging system with normalization by β-actin (B). *: P<0.05 Wnt3a vs. vehicle (n=4). (C and D) Effects of β-catenin/TCF4 on Wnt reporter activity. C2C12 cells, which were transfected with TOPFLASH (C) or9×6-OC-Luc reporter (D), were transfected with expression vectors for wild-type and mutant β-catenin and TCF4at a dose of 0.2 µg DNA/well in 24-well plates for 36 h. Relative luciferase activity in the cell lysates was measured and normalized by β-galactosidase (β-gal) activity. #: P<0.05 β-catenin or TCF4 vs. vector; *: P<0 .01 β-catenin+TCF4 vs. β-catenin (n=6). (E) Effects of DKK1 on β-catenin activation of Wnt signaling activity. C2C12 cells carrying TOPFLASH reporter were incubated with DKK1 at 100 ng/mL for 36 h. Relative luciferase activity was measured with normalization by Renilla. *: P<0 .01 β-catenin vs. vector; #: P<0.01 β-catenin vs. β-catenin+DKK1 (n=6). (F) Effects of Wnt3a on Wnt signaling activity. C2C12 cells carrying TOPFLASH reporter were incubated with Wnt3a in a dose range of 10–80 ng/mL for 36 h. Relative luciferase activity was measured as described in (E). *: P<0 .01, Wnt3a vs. vehicle (n=6).