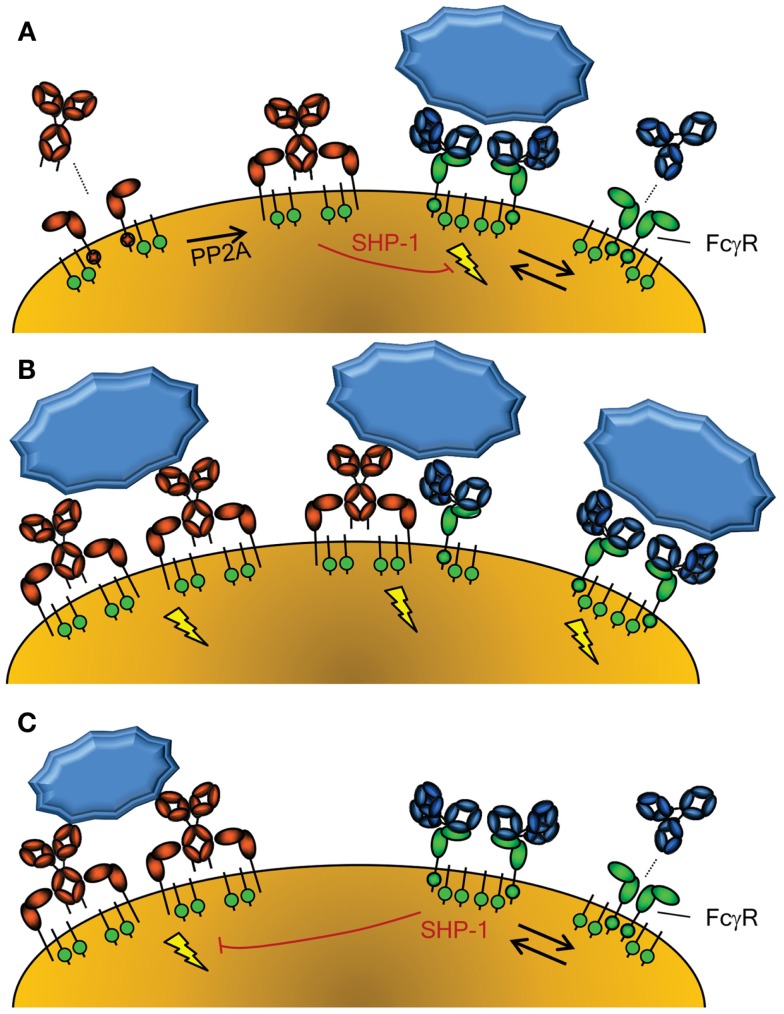
Figure 2.
Activation of cellular responses through Fc-Receptors. (A) The activity of FcαRI (left) is controlled through inside-out signaling through PP2A, that dephosphorylates the intracellular tail of FcαRI, enabling binding of IgA (58). Conversely, FcγR (right) seem to be continuously enabled, although crystallographic data suggest a dimeric form may exist that cannot interact with IgG without disrupting the inert FcγRIIa dimer (115). The FcγRIIa engaged by IgG may however form a higher order dimer, or multimer, with either another FcγR-IgG unit or unligated FcγR, forming an active signaling complex after engagement with IgG-opsonized target. However, without crosslinking of FcαRI through IgA and it’s cognate antigen, the FcαRI has been reported to lead to down regulation of FcγRI signaling through phosphorylated SYK (58). (B) Importantly, co-engagement of FcγR and FcαR results in a strong activation of phagocyte responses, with FcαRI leading to a more prominent respiratory burst activation, while FcγR result more a prominent phagocytosis response (54). (C) FcγR can also mediate inhibitory signal, because IgG-ligated, either by monomers at high concentrations, dimers, or F(ab’)2-anti FcγR, can also cause inhibition of other ITAM-, but also non-ITAM, depended cellular activation, also through phosphorylated SYK (60).