Figure 4.
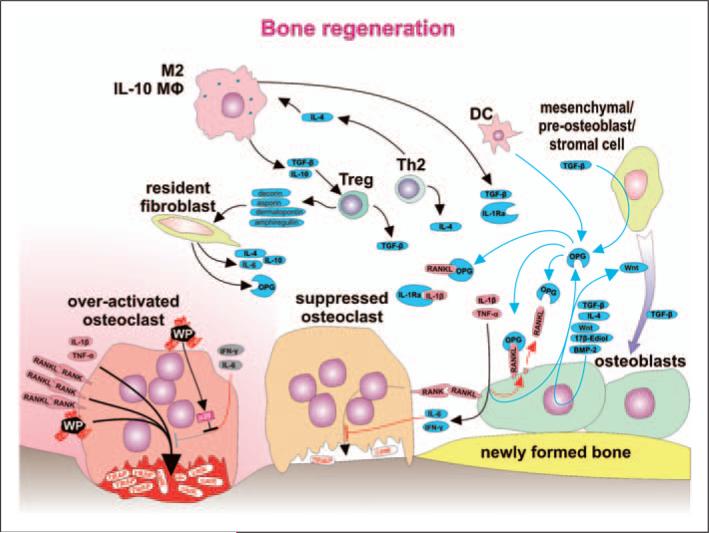
Bone regeneration is orchestrated with a substantial contribution from immune cells. Within the bone multicellular unit (BMU), suppression of the inflammatory response is associated with a change from net bone resorption toward bone remodeling. Osteoclasts stimulated by RANKL, IL-1β, TNF-α and wear particles pump protons (H+), move toward the bone surface and secrete bone destructing cathepsin K (catK) and tartarate-resistant acid phosphatase (TRAP). A decrease in the level of the stimulating factors is a consequence of anti-inflammatory activity of several immune cell types (M2, IL-10-secreting macrophages, DC, Treg cells, Th2 cells), as well as non-immune cells, such as resident-tissue fibroblasts and mesenchymal/pre-osteoblast/stromal cells. Anti-inflammatory activity is mediated by soluble factors IL-4, IL-10, TGF-β, soluble receptor for IL-1 (IL-1Ra), and OPG secreted and acting within the area of BMU. Strong immunosuppressive Treg cells differentiate under the influence of TGF-β and IL-10 secreted from M2 and IL-10-secreting macrophages and specific populations of DC. Further, Treg cells secrete decorin, asporin, dermatopontin and amphiregullin contributing to a reparative bone remodeling. Bone remodeling is further supported by stimulation of osteoblasts by 17β-estradiol, differentiation factor Wnt and bone morphogenetic protein 2 (BMP-2)—some of them acting as autocrine factors. IFN-γ secreted by Th1 and NK cells or IL-6 secreted by T cells and macrophages contribute to bone remodeling by activation of osteoblasts to produce RANKL inhibitor OPG. The number of osteoblasts increases as osteoblast precursors differentiate under the influence of sphingosine-1-phosphate produced by mature osteoclasts. The mechanisms above and TGF-β, together with other factors, contribute to the recruitment and differentiation of pre-osteoblasts toward mature bone remodeling osteoblasts. Furthermore, IFN-γ and IL-6 suppress bone resorption by acting directly upon osteoclasts. Nevertheless, activated osteoclasts are not responsive to IFN-γ and IL-6 because intracellular response pathways are blocked by over-expressed p38.