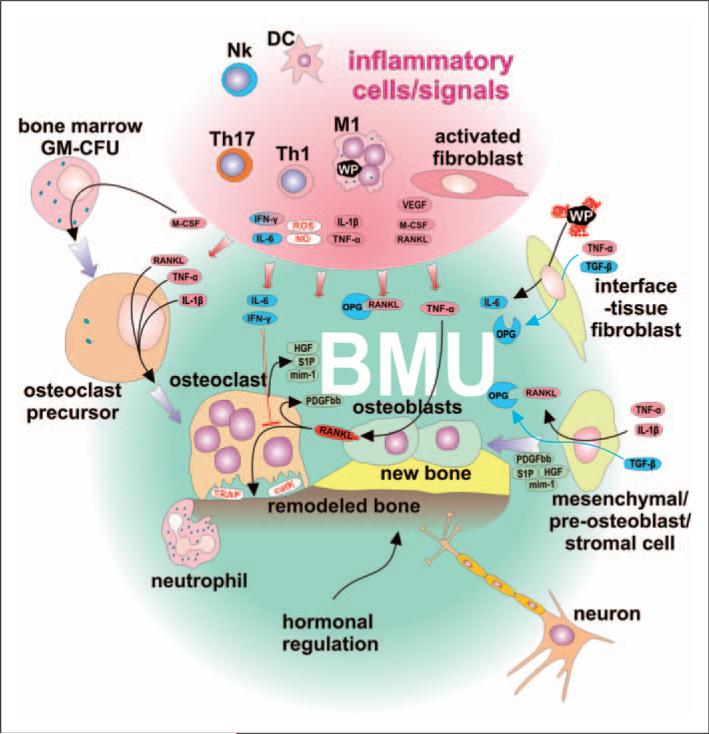
Figure 5.
The BMU is self regulatory and not affected by pro-inflammatory signals produced by activated immune cells and fibroblasts. Classically-activated M1 macrophages and subpopulations of DCs secrete Th1- and Th17-stimulating cytokines, as well as toxic (reactive) nitrogen and oxygen species, and COX-2. Stimulated Th1 lymphocytes secrete RANKL, one of the most important osteoclast-activating cytokines. M1 further secrete cytokines IL-1β and TNF-α, thus inducing pre-osteoclast differentiation and activation leading to increased bone resorption. IL-1β and TNF-α stimulate osteoblasts to secrete RANKL, thus creating positive feedback for bone resorption. Furthermore, inflammatory-activated fibroblasts support bone resorption by secretion of RANKL and stimulation of pre-osteoclast differentiation by M-CSF. Although the BMU is exposed to the above factors, it has its own regulatory mechanisms to help ensure homeostasis: the BMU is covered by a canopy of cells so that BMUs can undergo activation–resorption-formation cycles in bone remodeling compartments. Osteoclasts exposed to IL-6 and IFN-γ do not respond to RANKL-mediated activation. Furthermore, IL-6 is secreted by fibroblasts after exposure to wear particles. Osteoclasts, upon stimulation by RANKL, secrete platelet-derived growth factor bb (PDGFbb), which induces the proliferation of pre-osteoblasts leading to an increase in the number of osteoblast precursors. Furthermore, osteoclasts produce sphingosine 1-phosphate (S1P), myb-induced myeloid protein-1 (mim-1) and hepatocyte growth factor (HGF), collectively contributing to pre-osteoblasts migration and osteoblast survival. Attenuation of osteoclast activity leads to a decrease in the production of PDGFbb and S1P-induced differentiation of pre-osteoblasts toward active osteoblasts and bone remodeling. Physiologically-activated interface tissue fibroblasts/tissue-resident fibroblasts respond to TNF-α together with TGF-β by secretion of OPG. In addition, BMU neurons could sense local inflammation and modulate activity of both immune and BMU cells through ATP and neuropeptide mediators, and further activate the neuroendocrine system. All the above factors are most prominent early after total hip arthroplasty surgery or when the inflammatory response is more quiescent.