Figure 2.
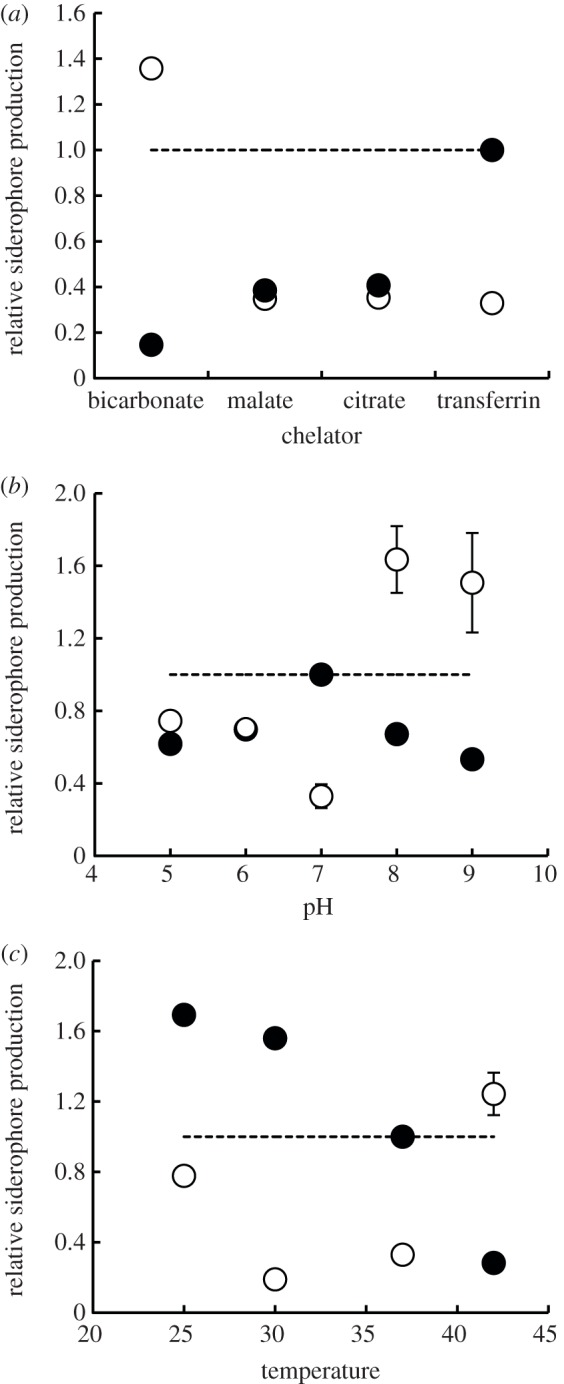
Siderophore switching as a function of relative iron availability in the environment. We manipulated relative iron availability in the medium by: (a) adding chelators with different iron-binding affinities (low to high from left to right); (b) varying the pH of the medium, which alters siderophore and/or chelator affinity to iron; and (c) varying incubation temperature, which determines the thermodynamic potential of chemical reactions and therefore the speed of iron uptake. Overall, there was a significant switch from the production of pyoverdine (filled circles) to pyochelin (open circles) when moving from environments with lower to higher relative iron availabilities. Here, the production of each siderophore is represented as a relative measure, i.e. the RFUsiderophore/OD, relative to the RFUpyoverdine/OD, measured under baseline conditions (i.e. transferrin, pH 7, 37°C). Values are given as mean±s.e. Some standard errors are smaller than symbols, and are therefore not shown.
