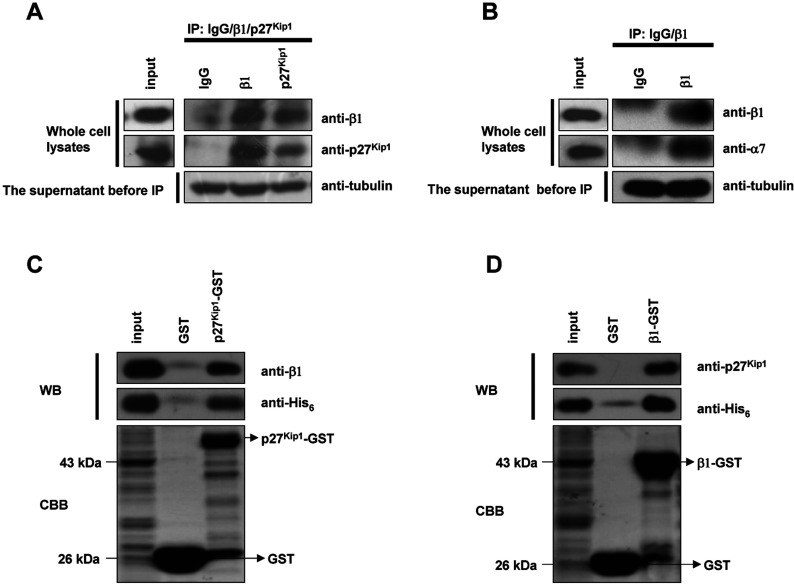
Figure 4. β1 interacts with p27Kip1 in vivo and in vitro.
(A) Immunoprecipitation analysis of the interactions between p27Kip1 and β1. Cell lysates from synchronized G0 HeLa cells were divided into three parts and each part was incubated with antibody (β1, GFP and p27Kip1 mouse monoclonal antibody) overnight and then with protein G-plus-agarose beads at 4°C for 4 h. The beads were washed three times with precipitation buffer and then treated, as above. For detection of interactions, the blot was probed with anti-β1/p27Kip1 rabbit polyclonal antibodies, followed by ECL. (B) Immunoprecipitation analysis of the interactions between β1 and the α7 subunit. For detection of this interaction, the blot was probed with anti-β1/α7 antibodies, followed by ECL. (C) GST-pull-down analysis of the interactions between p27Kip1 and β1. p27Kip1–GST fusion proteins were expressed by the addition of IPTG to 0.2 mM at 25°C for 4 h. Bacterial cells were lysed and the supernatants were incubated with the glutathione resin overnight and then with purified β1–His6 for 4 h at 4°C, followed by washing and elution. To detect interactions, the blots were probed with anti-β1/His6 antibodies, followed by ECL. The lower panel shows the amounts of bound GST and GST-tagged proteins by CBB (Coomassie Brilliant Blue) staining. (D) β1-GST fusion proteins were incubated with purified p27Kip1-His6 at 4°C for 4 h and then detected with anti-p27Kip1/His6 antibody, followed by ECL. The lower panel shows the amounts of bounded GST and GST-tagged proteins by CBB staining.