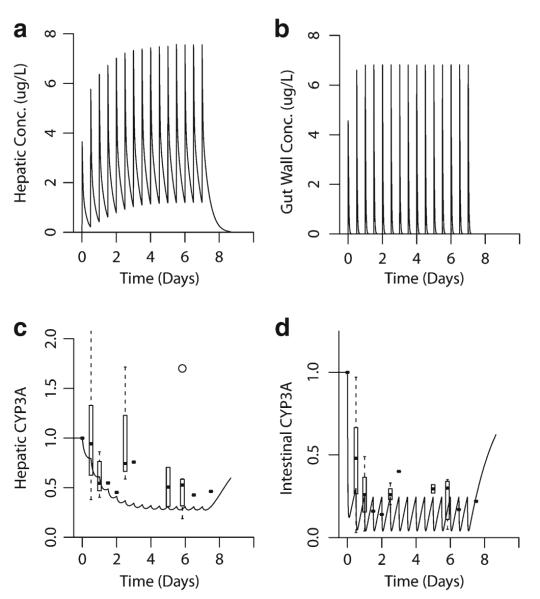
Fig. 6.
Simulated effect of clarithromycin on hepatic and intestinal CYP3A activity. A semi-physiologically based pharmacokinetic model incorporating effects of clarithromycin inactivation in the intestine and liver was developed. Clarithromycin 500 mg was administered orally every 12 h. a, b Simulated hepatic and gut-wall concentrations of clarithromycin. c Predicted (line) and observed (box plot/point estimates) hepatic CYP3A activity. d Predicted (line) and observed (box plot/point estimates) gut-wall CYP3A activity