Abstract
Background: Controversy exists regarding the causative role of dietary fructose in obesity and fatty liver diseases. Clinical trials have indicated that negative health consequences may occur only when fructose is consumed within excess calories. Animal studies have suggested that fructose impairs intestinal integrity and leads to hepatic steatosis (HS).
Objectives: We assessed nonhuman primates after chronic ad libitum and short-term calorically controlled consumption of a high-fructose (HFr), low-fat diet (24% of calories). Microbial translocation (MT), microbiome, and metabolic health indexes were evaluated.
Design: Seventeen monkeys fed 0.3–7 y of an HFr ad libitum diet were compared with 10 monkeys fed a low-fructose, low-fat diet (control). Ten middle-aged, weight-stable, fructose-naive monkeys were stratified into HFr and control groups fed for 6 wk at caloric amounts required to maintain weight stability. Metabolic endpoints, feces, liver, small and large intestinal biopsies, and portal blood samples were collected.
Results: Monkeys allowed ad libitum HFr developed HS in contrast to the control diet, and the extent of ectopic fat was related to the duration of feeding. Diabetes incidence also increased. Monkeys that consumed calorically controlled HFr showed significant increases in biomarkers of liver damage, endotoxemia, and MT indexes and a trend for greater hepatitis that was related to MT; however, HS did not develop.
Conclusions: Even in the absence of weight gain, fructose rapidly causes liver damage that we suggest is secondary to endotoxemia and MT. HS relates to the duration of fructose consumption and total calories consumed. These data support fructose inducing both MT and ectopic fat deposition in primates.
See corresponding editorial on page 264.
INTRODUCTION
Hepatic steatosis (HS)4 is the accumulation of ectopic fat in the liver and is an early stage in the nonalcoholic fatty liver disease group. HS has important consequences for metabolic health because it is associated with the development of diabetes (1–3) and cardiovascular disease (4, 5) conditions, which cumulatively account for the majority of mortality in the United States (US National Vital Statistics Survey 2010). HS affects 20–50% of Americans (6, 7) and, thus, poses a significant public health problem in terms of both morbidity and mortality. Although generalized weight gain of the population is believed to contribute to the high prevalence of HS, specific contributory factors are unknown. Dietary fructose is a candidate because its consumption has paralleled the obesity epidemic in the United States; however, significant controversy exists because iscaloric comparisons of fructose and other carbohydrate sources have failed to show obesigenic effects (8).
Studies in rodents and dogs have used fructose in dietary interventions to induce HS but often have not been optimally designed to conclude that fructose specifically induced fat deposition because many studies have been confounded by ad libitum feeding and consequential weight gain (9, 10). Uncontrolled human trials that have used food-frequency questionnaires have concluded that only the consumption of sweetened beverages predicted fatty liver (11) and implicated fructose as the predominant sugar in beverages currently consumed. The main objective of our study was to bridge this gap in knowledge by using a relevant nonhuman primate model of insulin resistance and diabetes development (12) to understand what high dietary fructose does to liver health under calorie-controlled and -uncontrolled conditions.
Microbial translocation (MT) is the passage of live bacteria or bacterial products present in the gastrointestinal tract to extraintestinal sites (13). There is a history of bacterial endotoxemia that is related to hepatic lipid accumulation (14–17), which has been supported by the observation that antibiotic use has decreased HS in models of bacterial overgrowth (18) and in relation to high fructose (HFr) feeding (19). In addition, the antibiotic reduction in the microbial content of diabetic mice improves glucose tolerance and HS in the absence of changes in body weight (15). Increased MT and endotoxemia have also been observed with high-fat diets (20) and where the gut microbiome has changed (14), which is a consequence of typical Western diets. Thus, an additional objective of our study was to assess MT in nonhuman primates that consumed dietary fructose in a low-fat context over a duration short enough that microbiomic changes were unlikely to have occurred (21).
MATERIALS AND METHODS
Animal experiments
All experimental procedures that involved animals were approved and complied with the guidelines of the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. Old world monkeys (Macaca fasciculus and Chlorocebus aethiops) were maintained in pairs and fed once daily either a control diet (Diet 5038, LabDiet; Purina) or an HFr diet that was constructed on site at the Wake Forest School of Medicine (Table 1). The control diet was a low-fat, high-carbohydrate diet. The carbohydrate content of the control diet was primarily grain starches and dietary fiber, with <3% of calories supplied as sucrose and glucose and <0.5% of calories supplied as fructose. The HFr diet was also low fat and had an equivalent carbohydrate content. In contrast to the control diet, 24% of calories were supplied from fructose in the HFr diet. All diets were complete and balanced and met the recommended guidelines for adult old world primates.
TABLE 1.
Nutritional information of experimental diets (control and HFr) used1
| Control | HFr | |
| Caloric density (kcal/g) | 3.19 | 3.37 |
| Carbohydrate (percentage of energy) | 69 | 69 |
| Fructose (percentage of energy) | <0.5 | 24 |
| Carbohydrate sources | Grain starches, plant fiber | Wheat flour, fructose |
| Protein (percentage of energy) | 18 | 14 |
| Protein sources | Whey, grain, and fish meals | Casein, lactalbumin, and wheat flour |
| Fat (percentage of energy) | 13 | 17 |
| Dietary fat sources | Pork fat | Vegetable oils, butter |
| MUFA (percentage of fatty acids) | 33 | 35 |
| SFA (percentage of fatty acids) | 30 | 34 |
| PUFA (percentage of fatty acids) | 37 | 30 |
| Dietary fiber (percentage of diet) | 4.5 | 3.8 |
| Cholesterol (ppm) | 75 | 67 |
HFr, high fructose; ppm, parts per million.
Study 1: chronic ad libitum fructose consumption
Seventeen male and female monkeys were identified from a larger cohort allowed to consume the HFr diet ad libitum for durations ≤7 y (∼20 y human equivalent). Animals analyzed in the study had died for reasons unrelated to any experimental intervention and, therefore, had liver histology available for examination. Ten monkeys deemed to have good general health and life-long exposure to the control diet were identified as having liver histology available after euthanasia for reasons unrelated to any study intervention. Sex distributions between groups were not significantly different with 71% of females in the HFr group compared with 50% of females in the control group (P = 0.06).
Liver tissue was fixed and embedded, and sections were stained with hematoxylin and eosin. Two blinded individuals grossly scored steatosis presence by counting lipid vacuoles from 10 fields that surrounded the portal triad at a magnification ×40. Microsteatosis was noted and arbitrarily assigned a count of one for every 5 intracytoplasmic lipid vesicles.
Study 2: subacute calorically controlled fructose consumption
Study design
Ten female, middle-aged to aged monkeys that had been maintained on the control diet were included in study. They were stratified into 2 groups on the basis of age and body weight with calories supplied at 70 kcal · kg−1 · d−1, which is an amount known to promote weight stability (22). Bodyweights were recorded weekly and individual caloric intakes were adjusted to avoid any weight gain or loss for a period of 1 mo before study. At baseline and after 6 wk of controlled dietary intake of either the control or HFr diet, monkeys were sedated with ketamine (15 mg/kg intramuscularly) for blood sampling, recording of waist circumference, and blood pressure measurements. After 6 wk of control- or HFr-diet exposure, all monkeys were separately sedated with ketamine and anesthesia maintained with isoflurane to facilitate surgical collection of liver and small intestinal biopsies and blood sampling from the portal vein.
Blood and biochemical endpoints
Blood samples were processed, and clinical chemistry endpoints were measured, from serum at a commercial veterinary laboratory (Antech Diagnostics). Glycation of blood hemoglobin A1c was measured by using HPLC (Primus PDQ; Primus Diagnostics) to assess long-term glycemic control. Insulin was measured by using an ELISA (Mercodia), and HOMA-IR values were calculated by the product of insulin concentrations as units per liter and glucose concentrations as millimoles per liter divided by 22.5. Liver lipids and triglycerides were extracted and measured as previously described (23). Total and free cholesterol concentrations were determined enzymatically. The esterified cholesterol concentration was calculated as the difference between total cholesterol and free cholesterol. Results are expressed as micrograms of lipid per milligram of protein measured in the liver sample.
Pathologic assessments
Liver tissue was immersion fixed in 10% neutral formalin for paraffin embedding, and sections were stained with hematoxylin and eosin. The liver was also snap frozen and sectioned on a freezing microtome for oil red O staining (Sigma-Aldrich) to assess neutral lipid in situ. Sections were assessed by a board certified veterinary pathologist blinded to group assignments of monkeys. A pathologic score (0–4) for liver inflammation was developed with a score of 0 indicating no inflammation, a score of 1 indicating minimal portal inflammation with ≥30% of portal triads affected, a score of 2 indicating mild portal inflammation with ≥30% of portal triads affected, a score of 3 indicating moderate inflammation with ≥30% of portal triads affected, and a score of 4 indicating moderate inflammation with foci of piecemeal inflammation and necrosis. Sections that were oil red O stained were quantified by color-imaging software (Image Pro Plus, Version 5.1; Media Cybernetics). The area of all tissue was measured by a color selection that corresponded to hematoxylin (A). The area of positively stained lipid was measured by a color selection that corresponded to the red (B), and the percentage of staining was expressed as
The intestinal integrity was assessed in biopsy specimens by using immunostaining and scoring for zona occludens-1, occludin, and claudin-1 protein intensity and immunoblotting for occludin protein amounts (for a description, see Table 1 and Figures 1 and under “Supplemental data” in the online issue).
FIGURE 1.
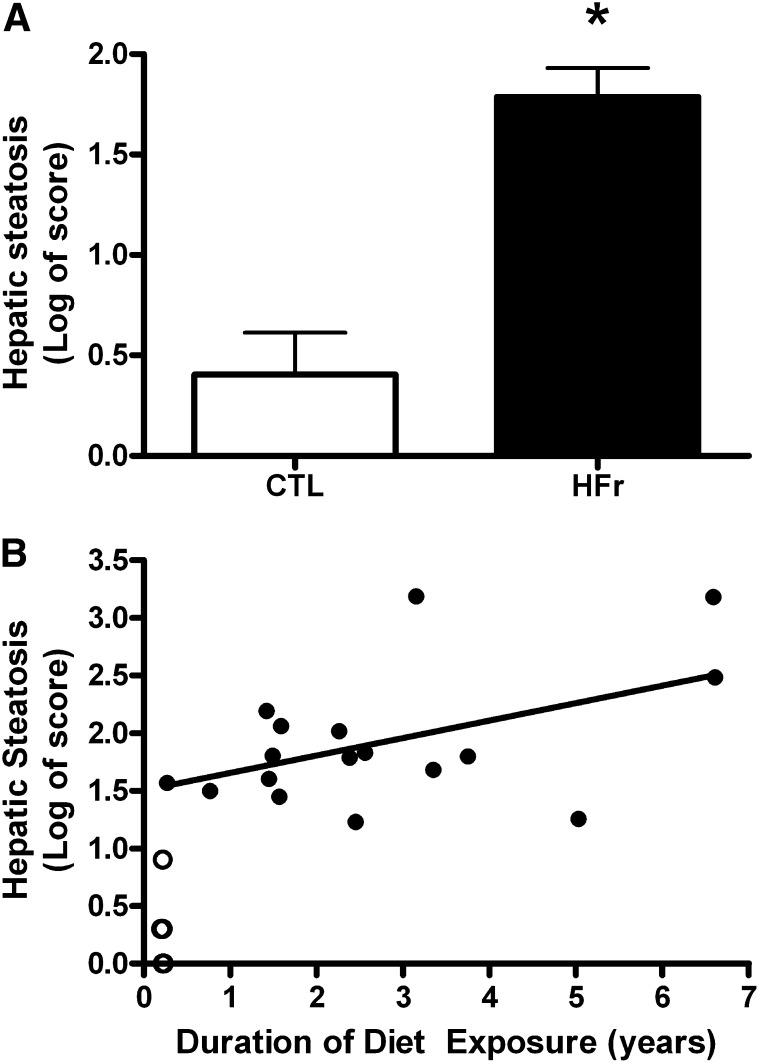
A: Least-squares mean (±SEM) hepatic steatosis scores adjusted for diet duration, age, and body weight. Scores were calculated from a histologic section examination by duplicate blind reviewers from monkeys fed an ad libitum CTL (n = 10) or HFr (n = 17). The HFr induced greater liver fat deposition (ANCOVA; *P < 0.001). B: Scatterplot of hepatic steatosis scores and duration of consumption of either the CTL (n = 10; open circles) or HFr (n = 17; closed circles). The partial correlation coefficient reflects adjustment for age and body weight (r = 0.61, P < 0.05). Monkeys that ate the CTL had lifelong exposure but are represented at 0.2 y, which reflects the time that they were housed at the same site as HFr monkeys. The majority of these CTL monkey values are superimposed with a steatosis score of 0, and thus, the 10 animals are not discriminated on the graph. CTL, low-fat, low-fructose diet; HFr, low-fat, high-fructose diet.
MT indexes
Plasma harvested from anticoagulated portal blood samples was stored at −80°C until analysis. Bacterial endotoxin concentrations were quantified by using the kinetic chromogenic limulus ameobocyte lysate assay for LPS (Lonza) according to the manufacturer's recommendations with the following modifications. Plasma was diluted 1:1000 with magnesium chloride and heated to 70°C for 30 min before assay. The average recovery rate for this assay was 96%. Soluble CD14, LPS binding protein, and C-reactive protein concentrations were measured by using an ELISA in plasma from the peripheral circulation (R&D Systems, Hycult Biotech, and ALPCO Diagnostics, respectively).
Microbiomic analyses
Weekly fecal samples were collected either from collection pans under the individual's housing or by gentle manual extraction while sedated. An 85% fecal recovery rate was achieved. Feces were frozen in liquid nitrogen until DNA extraction by using the Qiagen QIAamp stool mini kit (Qiagen) according to the manufacturer's protocol. Eluted DNA was quantitated by using UV spectrometry with a Nano-Drop ND-1000 spectrophotometer (Thermo-Scientific). Samples were prepared for 16D ribosomal RNA gene sequencing (Illumina) targeting the V6 hypervariable region, and resulting sequences were clustered into operational taxonomic units and analyzed by using principal coordinate analysis for group differences (see Online Supplemental Material under “Supplemental data” in the online issue for details of full methods).
Data analysis
Data are presented as means ± SEMs for each group. Data were analyzed for normality and logarithmically transformed where necessary before ANCOVA (with adjustment for age, body weight, baseline value if available, and diet duration if applicable) was performed to assess for group differences. Pairwise associations between variables were evaluated by using Pearson's correlation coefficient if normally distributed, and Spearman's rank order correlation for categorical data. Statistical analysis was performed with Statistica v10 software (StatSoft Inc). Significance was set at α ≤ 0.05 for group differences and α ≤ 0.1 for trends.
RESULTS
Study 1: chronic ad libitum fructose consumption
In this study, 17 male and female monkeys were allowed to consume an ad libitum low-fat, high-fructose diet for ≤7 y and were compared with 10 monkeys that consumed a low-fat, low-fructose diet (see Materials and Methods). Compared with control monkeys, fructose-fed monkeys had significantly heavier body weights (3.03 ± 0.09 compared with 5.42 ± 0.83 kg, respectively; P = 0.04) and were older (14.4 ± 2.53 compared with 22.36 ± 2.21 y, respectively; P = 0.01). Of >70 monkeys exposed to the fructose diet over the 7-y period examined, 15% of monkeys developed overt type 2 diabetes, which is 3 times the incidence rate historically observed at the Wake Forest School of Medicine primate facility in monkeys who consumed a fructose-free diet. In addition, the median duration of HFr-diet consumption required to induce type 2 diabetes was 3.7 y (or equivalent to ∼12 human years). HS scores were significantly higher in HFr monkeys (P < 0.001; Figure 1A), and these scores correlated strongly with the duration of diet consumption (r = 0.61, P < 0.05; Figure 1B). Note that even the shortest duration of ab libitum feeding (3 mo) of the HFr diet resulted in elevated liver lipid counts (Figure 1B). Control monkeys had lifelong exposure to the low-fat, low-fructose diet but were represented graphically with all data points at 3 mo duration, which reflected the time they were housed at Wake Forest School of Medicine. To examine the relative contributions of age, weight gain, duration of consumption, sex, and experimental diets fed on HS, a multiple regression analysis was conducted, with the HS score as the outcome variable. The overall model was highly significant (R2 = 0.81, P < 0.001) with only diet type (HFr compared with control) and diet duration retained as significant predictors. Diet had the greater magnitude of effect (β = 0.6, P < 0.0001) than that of the duration of diet consumption (β = 0.15, P = 0.02) on the development of HS.
Study 2: subacute calorically controlled fructose consumption
In this experiment, monkeys of similar age (15.2 ± 2.3 y in the control group compared with 11.9 ± 2.3 y in the HFr group; P = 0.39) were exposed to a high-fructose, low-fat or low-fructose, low-fat diet for a period of 6 wk. Caloric intake of the 2 diets was successfully controlled to prevent significant weight changes in either group (Table 2). To achieve weight stability, on average, monkeys that ate the control diet were fed 77 kcal · kg−1 · d−1, whereas HFr diet fed monkeys were fed 67 kcal · kg−1 · d−1. The difference was attributed to the increased palatability of the HFr diet that resulted in less wastage of the supplied diet. Correspondingly, there were no overall changes in metabolic syndrome variables measured (Table 2). Thirty-seven percent higher total plasma cholesterol concentrations were noted in the HFr group. Both insulin and glucose concentrations were higher, on average, in the HFr diet–fed group at the end of study, and thus, HOMA values were also higher.
TABLE 2.
Metabolic variables measured in monkeys before and after controlled intake of low-fat diets that were either fructose free (control; n = 5) or HFr (n = 5)1
| Baseline | 6 wk | ANCOVA P | |
| BMI (kg/m2) | 0.88 | ||
| Control | 28.7 ± 2.84 | 28.5 ± 2.31 | |
| HFr | 28.7 ± 3.23 | 28.3 ± 3.23 | |
| Body weight (kg) | 0.66 | ||
| Control | 5.41 ± 0.40 | 5.54 ± 0.39 | |
| HFr | 5.25 ± 0.63 | 5.44 ± 0.73 | |
| Waist circumference (cm) | 0.87 | ||
| Control | 36.2 ± 2.6 | 36.4 ± 2.2 | |
| HFr | 36.5 ± 4.7 | 36.5 ± 4.8 | |
| Glucose (mg/dL) | 0.35 | ||
| Control | 30 ± 10 | 34 ± 8 | |
| HFr | 45 ± 14 | 74 ± 24 | |
| Insulin (U/L) | 0.43 | ||
| Control | 15.7 ± 1.76 | 28.9 ± 6.40 | |
| HFr | 22.2 ± 4.79 | 41.9 ± 7.51 | |
| Hb A1c (%) | 0.81 | ||
| Control | 2.6 ± 0.03 | 2.7 ± 0.05 | |
| HFr | 3.1 ± 0.4 | 3.2 ± 0.5 | |
| HOMA-IR | 0.34 | ||
| Control | 1.28 ± 0.55 | 2.67 ± 0.90 | |
| HFr | 2.77 ± 1.11 | 9.08 ± 4.86 | |
| TPC (mg/dL) | 0.04 | ||
| Control | 149 ± 13 | 145 ± 10 | |
| HFr | 162 ± 10 | 221 ± 28 | |
| Triglyceride (mg/dL) | 0.15 | ||
| Control | 47 ± 7 | 44 ± 4 | |
| HFr | 49 ± 10 | 76 ± 29 | |
| SBP (mm Hg) | 0.85 | ||
| Control | 145 ± 16 | 124 ± 12 | |
| HFr | 107 ± 4 | 100 ± 4 |
All values are means ± SEMs. P values represent differences between groups at 6 wk with the baseline value used as a covariate. All blood variables except Hb A1c were measured in plasma. Hb A1c, glycated hemoglobin; HFr, high fructose; SBP, systolic blood pressure; TPC, total plasma cholesterol.
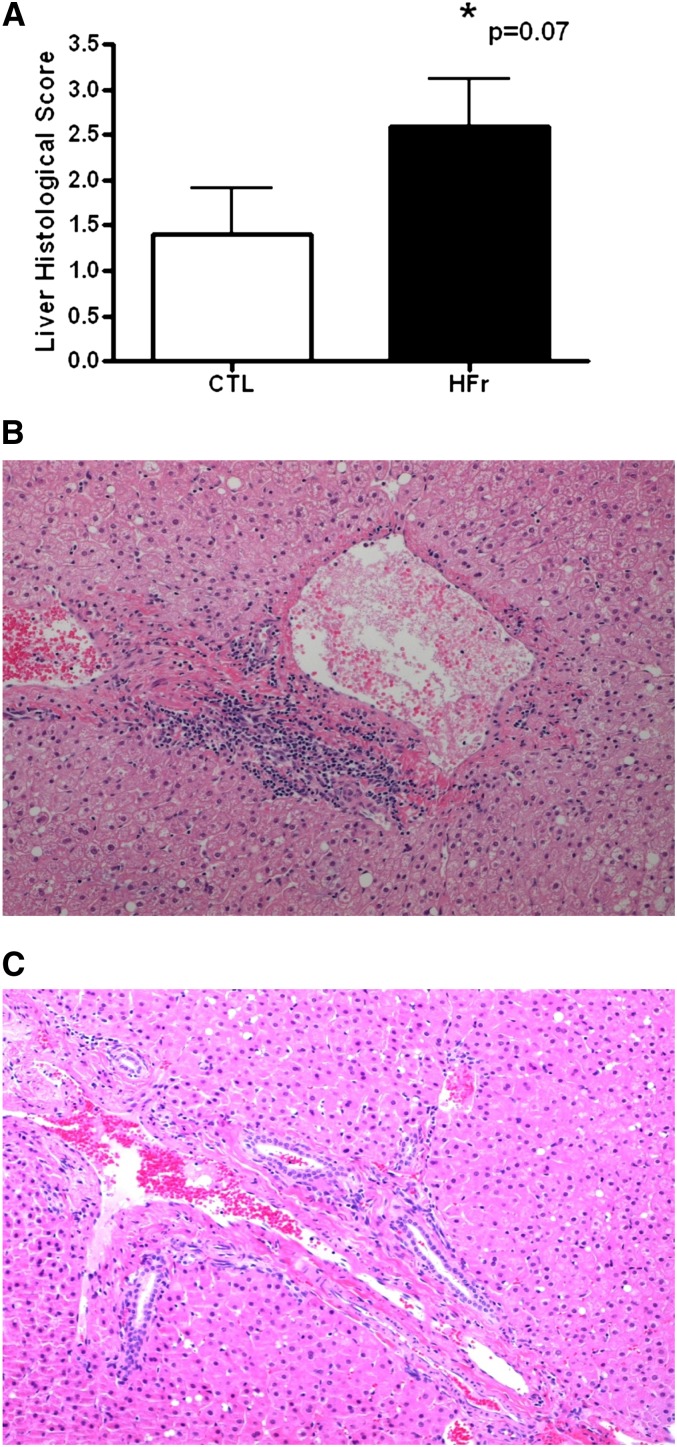
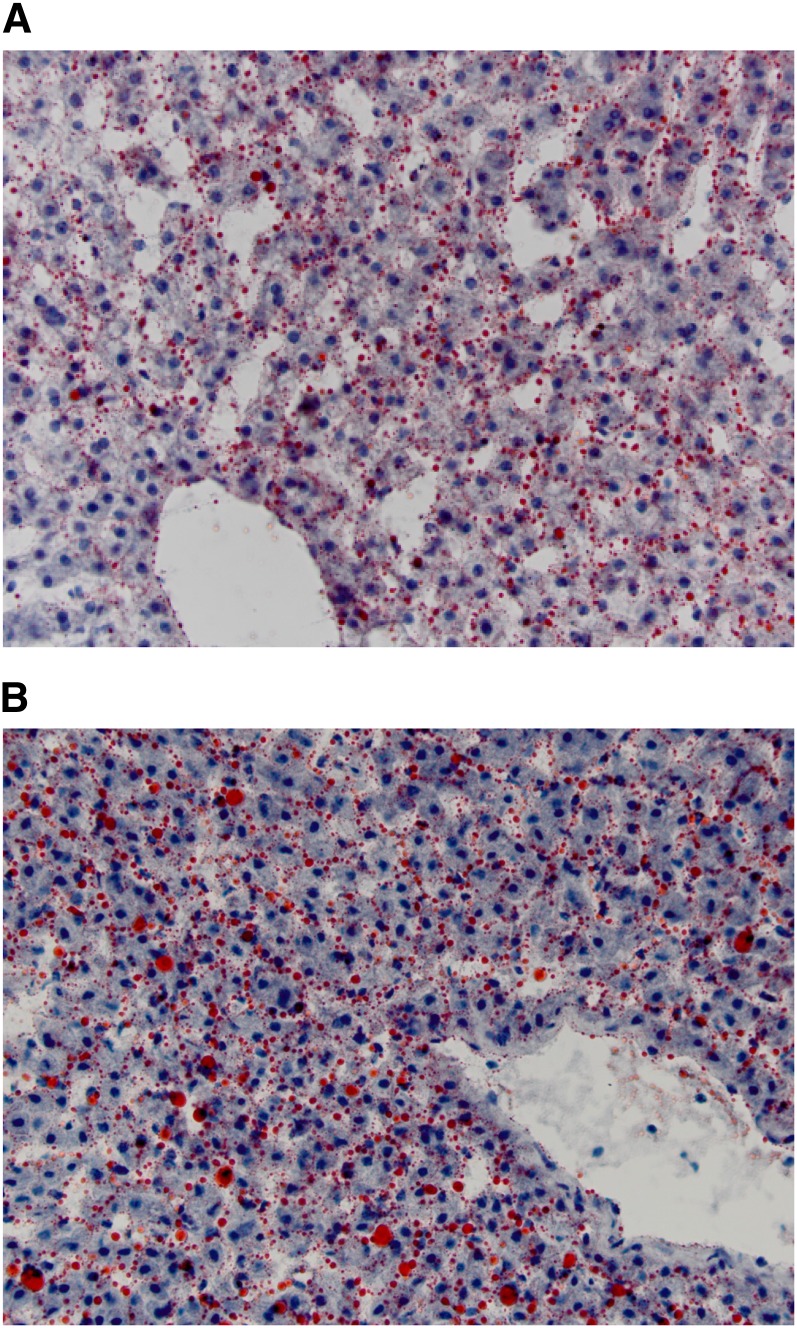
Despite the stability in weight and metabolic variables, hepatic health as assessed by routine serum biochemistry showed that a calorically controlled supply of fructose in the diet was associated with a trend toward histologic liver damage (P = 0.07) even over this short 6-wk time period (Figure 2A). The lesions characterized in the HFr group were periportal and inflammatory, without evidence of necrosis or fat accumulation (Figure 2B). The lack of HS was confirmed by a biochemical analysis of liver lipids that indicated no significant group differences were present after 6 wk of dietary intervention (Table 3). A trend toward elevated cholesterol esters was apparent in the HFr monkeys. Quantification of the histologic staining of neutral lipids (Figure 3) also confirmed that HFr consumption did not induce fat deposition in the liver when calories were controlled (4.66 ± 1.77 compared with 3.38 ± 1.04; P = 0.38). Despite the lack of fatty liver, fructose-fed monkeys developed significant inflammation (Table 4) with large elevations apparent in the majority of liver enzymes measured and the acute-phase protein C-reactive protein. These data substantiated the histologic inflammatory infiltrates noted in the liver sections from fructose-fed monkeys. No differences in large intestinal histology were observed (data not shown; P = 0.34).
FIGURE 2.
A: Mean (±SEM) scores for liver pathology as assessed by histologic grading for inflammation from monkeys fed either a CTL (n = 5) or HFr (n = 5) for 6 wk. An ANCOVA P value is shown. B: Representative histologic section from a monkey fed the HFr (histologic score: 3) showing the periportal inflammatory infiltrate seen in fructose-fed monkeys. C: Representative histologic section from a liver biopsy taken from a CTL monkey (histologic score: 0). CTL, low-fat, low-fructose diet; HFr, low-fat, high-fructose diet.
TABLE 3.
Lipids measured from liver biopsy specimens after 6 wk of controlled intake of low-fat diets that were either fructose free (control; n = 5) or HFr (n = 5)1
| Triglyceride | Total cholesterol | Cholesterol esters | Free cholesterol | Phospholipid | |
| μg/mg protein | μg/mg protein | μg/mg protein | μg/mg protein | μg/mg protein | |
| Control | 35 ± 10 | 23 ± 2.2 | 4.1 ± 0.7 | 18 ± 1 | 187 ± 7.5 |
| HFr | 50 ± 7 | 26 ± 3.4 | 6.3 ± 0.6 | 21 ± 1.8 | 174 ± 5.1 |
| ANOVA P | 0.12 | 0.51 | 0.06 | 0.12 | 0.22 |
All values are means ± SEMs. HFr, high fructose.
FIGURE 3.
Representative liver histologic sections stained for lipid with oil red O. Low-fat, high fructose–fed monkeys (A) had similar lipid amounts quantified by histology and direct biochemical analysis as did low-fat, low-fructose–fed monkeys (B) (4.66% compared with 3.48% by area, respectively; ANCOVA P = 0.38).
TABLE 4.
Biochemical indicators of liver injury and inflammation in monkeys before and after controlled intake of low-fat diets that were either fructose free (control; n = 5) or HFr (n = 5)1
| Baseline | 6 wk | Percentage of change | ANCOVA P | |
| Alanine transferase (U/L) | 0.006 | |||
| Control | 61.8 ± 13.92 | 71.0 ± 24.6 | 15% | |
| HFr | 75.0 ± 16.3 | 286 ± 51.6 | 281% | |
| Alkaline phosphatase (U/L) | 0.002 | |||
| Control | 98.6 ± 12.4 | 98.8 ± 12.2 | 0% | |
| HFr | 91.4 ± 9.39 | 147.9 ± 12.1 | 64% | |
| γ-Glutamyl transpeptidase (U/L) | 0.0002 | |||
| Control | 37.0 ± 3.62 | 32.2 ± 3.64 | −14% | |
| HFr | 36.2 ± 6.82 | 84.0 ± 17.3 | 133% | |
| Aspartate transferase (U/L) | 0.29 | |||
| Control | 46.0 ± 6.13 | 41.0 ± 5.10 | −11% | |
| HFr | 48.8 ± 6.60 | 65.4 ± 11.7 | 34% | |
| C-reactive protein (ng/mL) | 0.02 | |||
| Control | 9.30 ± 2.45 | 6.75 ± 1.90 | −27% | |
| HFr | 8.30 ± 4.07 | 14.6 ± 5.23 | 43% |
HFr, high fructose.
Mean ± SEM (all such values).
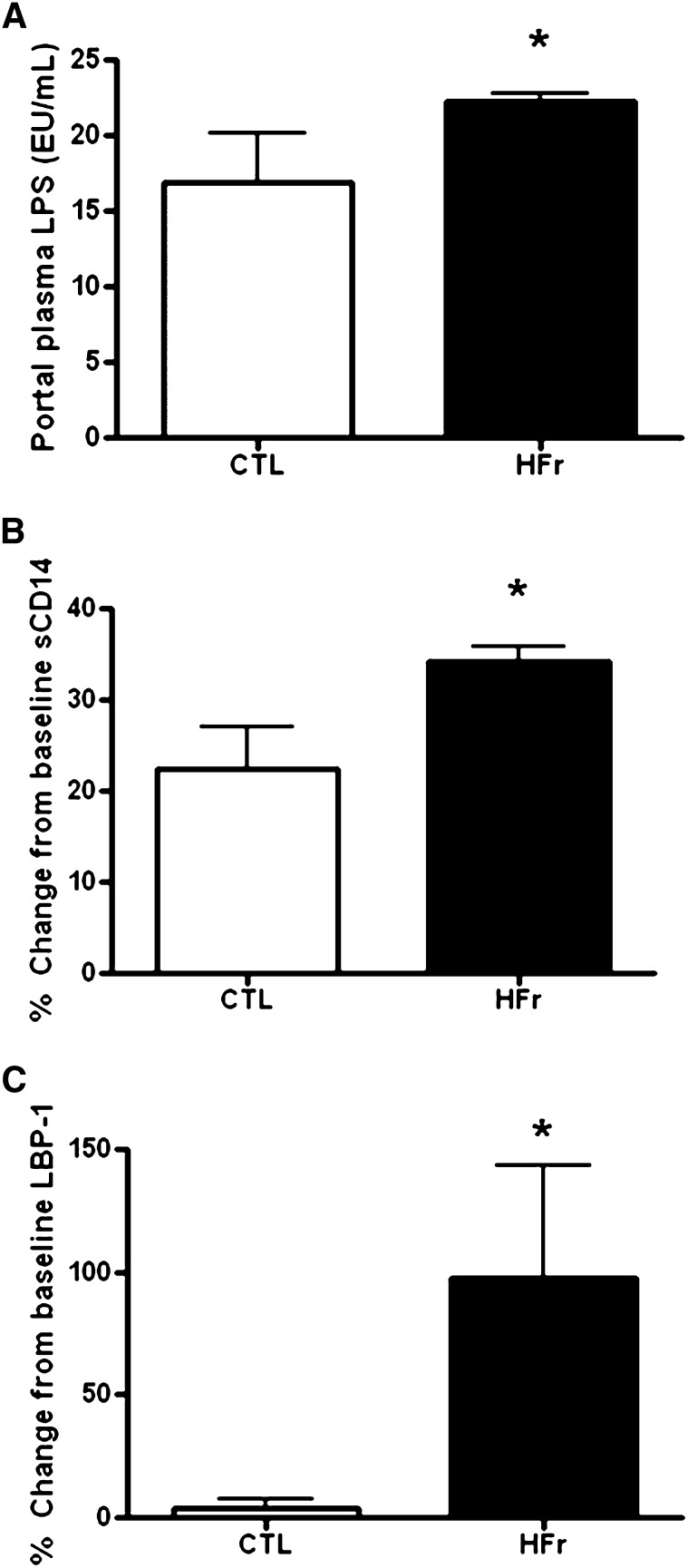
MT was directly measured by quantifying endotoxin in plasma taken from the blood draining the gastrointestinal tract to estimate what inflammatory stimulation the liver tissue was exposed to. We saw 31% higher endotoxin concentrations after short-term fructose feeding under calorically controlled conditions (Figure 4A; P = 0.05). Indirect measures of MT included significantly higher soluble CD14 (Figure 4B, P = 0.02) and LPS binding protein-1 (LBP-1) (Figure 4C; P = 0.04) measured in peripheral plasma from the HFr group, which supported the increase in translocation of microbial products with this diet (Figure 3, B and C). The role of MT and liver injury was further supported by the very strong associations between the change in LBP-1 concentrations and both liver damage (as measured by the change in alanine transferase concentrations; Pearson's r = 0.89, P = 0.001) and inflammation (as measured by the change in C-reactive protein concentrations; Pearson's r = 0.70, P = 0.02). In addition, the histologic inflammatory score tended to be associated with LPS (Spearman's r = 0.54, P = 0.11) and the change in concentrations of its ligand soluble CD14 (Spearman's r = 0.57, P = 0.08). Microbiomic analysis of fecal samples supported that this acute liver injury occurred in the absence of any differences in the microbiomic composition that resulted from diet exposure because animals exposed to the HFr diet did not have a distinct signature associated with the microbial community compared with in animals exposed to the low-fructose diet (Figure 5, A and B). After 6 wk, occludin protein was quantified in both the small and large intestine as a biomarker of intestinal integrity and was comparable between control and HFr monkeys (see Supplemental Figure 1 under “Supplemental data” in the online issue; P = 0.95 and P = 0.53, respectively). Immunostaining of small and large intestinal biopsy specimens for zona occludens-1, claudin-1, and occludin did not show significant differences (see Supplemental Table 1 under “Supplemental data” in the online issue; P < 0.05 for all); however, scores for all 3 of these tight junction proteins in the large intestine were lower in fructose-fed monkeys (see Supplemental Figure 2 under “Supplemental data” in the online issue).
FIGURE 4.
A: Endotoxin concentrations representing microbial translocation were measured from portal plasma samples taken after 6-wk consumption of controlled intakes of either a CTL (n = 5) or HFr (n = 5). *ANCOVA P = 0.05. B: The ligand for LPS (sCD14) significantly increased in the peripheral circulation after only 6 wk in monkeys fed the HFr. *ANCOVA P = 0.02. C: The binding protein for LPS (LBP-1) significantly increased in the peripheral circulation after only 6 wk in monkeys fed the HFr. *ANCOVA P = 0.04. CTL, low-fat, low-fructose diet; HFr, low-fat, high-fructose diet; LBP-1, LPS binding protein-1; sCD14, soluble CD14.
FIGURE 5.
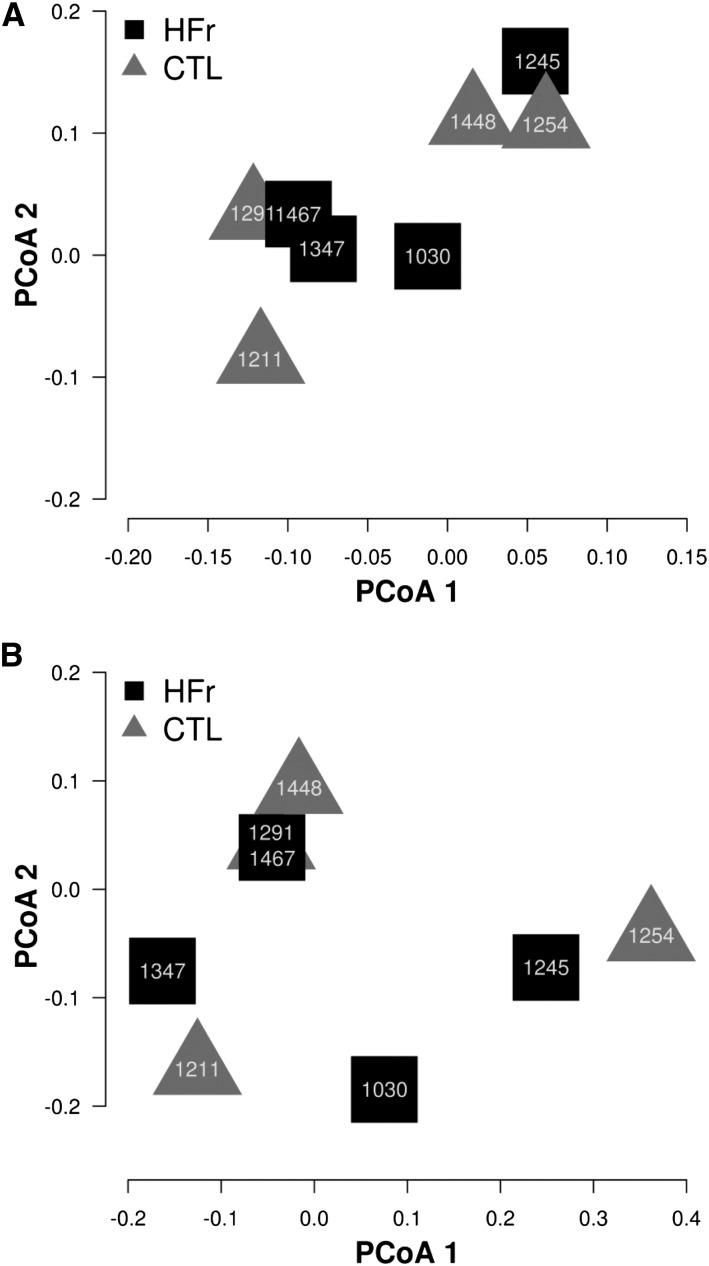
Cluster diagram on the basis of a PCoA of the fecal microbiome. Animal identification numbers are shown on the graph. A: No differences were observed between monkeys fed the CTL (triangles; n = 5) and those fed the HFr (squares; n = 5) at baseline (P = 0.89 for PCoA 1 and P = 0.91 for PCoA 2). B: No differences were also observed at the study end (P = 0.92 for PCoA 1 and P = 0.48 for PCoA 2). CTL, low-fat, low-fructose diet; HFr, low-fat, high-fructose diet; PCoA, principal coordinate analysis.
DISCUSSION
Our studies in nonhuman primates showed that high dietary fructose consumption rapidly induced changes in the liver, and these changes were likely secondary to increased endotoxemia and MT. Liver damage and inflammation were seen independently of changes in total adiposity, hepatic fat deposition, consumption of high-fat diets, substantial changes to the microbial community, and excess caloric consumption. Nonhuman primates readily develop HS and metabolic diseases such as diabetes when consuming an HFr diet; however, caloric excess and longer exposures were required for these phenotypes to manifest (24–26). Our study suggests that, in the most relevant animal model of human metabolic disease, 2 processes result from the current Western dietary environment; first, HFr induces MT and liver injury, and second, caloric excess as fructose promotes lipogenesis, which together promote the development of fatty liver diseases. These findings are in contrast to recent suggestions that lipogenesis may initiate fructose-associated nonalcoholic fatty liver diseases (27).
Clinical dietary studies in people are difficult to adequately control, and significant controversy still exists regarding whether fructose specifically induces hepatic and metabolic disease or whether increasing caloric intake is to blame. Recent clinical trials and meta-analyses (8, 28, 29) indicated that isocaloric clinical trials of fructose do not appear to be obesigenic or diabetogenic. The studies examined a range of fructose doses that extended above the supplementation used in the nonhuman primate studies and were of similar length to the current study. Our data showed that metabolic syndrome variables were not yet significantly affected by dietary fructose under isocaloric conditions and are consistent with these meta-analyses. However, glycemic endpoints and plasma triglycerides were all negatively impacted by short-term fructose, which was a result that was consistent with controlled clinical trials that evaluated fructose as 15–25% of energy and used more-sensitive measures to detect worsening glucose tolerance and insulin sensitivity (30–32). Nonsignificant changes seen in screening glycemic endpoints were consistent with the diabetogenesis observed in our nonhuman primates when given free access to the HFr diet, and our 15–20% incidence rate observed across both studies was remarkably similar to that observed in rhesus macaques supplemented with fructose at the same amount (24). The supplementation amount used in our study was comparable to the 95% level of intake of adolescent US males (33), which has been associated with elevated HOMA-IR values in this age group (3, 34). A study of the hepatic effects of HFr feeding in dogs confirmed that a 13-wk diet rendered the liver incapable of a normal glucose uptake and response to insulin and an inability to suppress gluconeogenesis (9), which contributed to the hyperglycemia and hyperinsulinemia that developed.
Our study uniquely showed the rapid and significant damage that occurred in the liver after HFr consumption in the absence of caloric excess. In some studies, fructose consumption and sweetened beverages have been associated with hepatic fibrosis (35) and fatty liver (11) in people, but these studies could not indicate causation and did not address total caloric intake. Rodent studies that used ad libitum sweetened water have shown that only fructose, and not sucrose or glucose, resulted in a larger liver (19). The increased liver size was related to a higher fat content and was seen without concomitant increases in body weight (19). In a separate study, liver weights and triglyceride contents were comparable in mice given access to sweetened water in combination with a high-fat diet; however, indexes of liver damage and fibrosis were greater in fructose-fed mice than in controls (10). Bergheim et al (19, 36–38) have generated an excellent group of studies that showed that ad libitum fructose supplementation led to enhanced intestinal permeability, endotoxemia, and increased indirect measures of MT in mice. We extend their findings to a more relevant animal model of human disease and provide a nutritional setting where the dietary components ingested were more similar to current westernized human diets. The finding that LPS, soluble CD14, and LBP-1 concentrations were all increased, whereas animals were weight stable and maintained on a low-fat diet, implicated simple sugars such as fructose in the creation of endotoxemia because endotoxemia has also been recognized as a feature of generalized obesity, insulin resistance, and secondary to the consumption of a high-fat diet (14, 39, 40). In addition, saturated fatty acids are known to be Toll-like receptor 4 activators (41). Thus, high–saturated-fat diets have the potential to induce inflammatory responses directly or via the induction of insulin resistance and elevation of circulating free fatty acids that results. Microbial translocating processes occur at a low rate normally; however, innate immune defenses remove these bacteria effectively through the processing of immune cells in the mucosal lamina propria or delivery of bacteria via the draining lymph to regional lymph nodes (42). Our data indicated that greater MT was seen without changes in the intestinal integrity and microbiomic profile such that an altered immune response and greater quantity of bacteria being presented to the mucosal surface or chylomicron incorporation could be hypothesized as the mechanisms. Periportal inflammatory infiltrates in the liver suggested that bacterial products are overwhelming local and innate immune mechanisms and being presented to Kupffer cells and nonimmune liver cells. All cells are able to respond to LPS by binding to Toll-like receptors and initiate inflammatory signaling (43); however, Kupffer and stellate cells are presumed to drive the fibrotic processes within the liver in more-advanced stages of disease (27, 35).
Western diets typically contain cholesterol, which may potentiate effects of fructose on hepatic de novo lipogenesis (44) and, thus, fructose-associated HS development. Our study included diets that were cholesterol deficient ,and this factor may have contributed to the lack of differences seen in the hepatic lipid content as assessed both biochemically and histologically. The findings presented in this study in nonhuman primates have implications for patients with preexisting liver diseases, and as has been suggested with fatty liver (35), that HFr and high-cholesterol diets should be avoided.
Our study was limited by a small sample size; however, our results showed large effects such that the statistical power was adequate to see group differences. Although results from this study have been ascribed to fructose in the diet, comparison with a glucose-fed group would have allowed our conclusions to be more specific. Data from human (30, 32) and rodent (19) studies that have compared the 2 simple sugars directly have shown that biologically significant differences exist with fructose on insulin sensitivity and adiposity. These studies all included an ad libitum component to their dietary trial interventions, and thus, our results support fructose-related differences even with controlled intakes and further substantiate MT as an initiating mechanism. Fructose has been postulated to alter the microbiome (45) through exposure to excessive amounts of sugar substrates in the Western diet and the interplay between bacterial species, which have a differing affinity for these diet components. In humans, microbiomic shifts secondary to diet changes occur only after chronic consumption (46), and thus, it was not surprising that our 6-wk study documented a stable microbiome within individuals. Nonhuman primates, such as human primates, potentially have fructose malabsorption that may modify the intestinal barrier function. We observed no change in the stool quality or diet acceptance over the study, suggesting fructose tolerance.
In conclusion, higher exposures to dietary fructose result in the development of hepatic lipidosis in nonhuman primates when consumed ad libitum for periods equivalent to ≥1 human year. In contrast, under conditions when the caloric intake is controlled, high dietary fructose does not induce changes in microbiome, adiposity, or metabolic syndrome criteria in the short-term; however, significant liver injury occurs with the initiation of inflammation. Hepatitis is related to greater endotoxemia and indirect measures of MT and supports the emerging primary mechanism by which dietary fructose may be associated with metabolic disease.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—KK: designed the study, conducted the research, analyzed data, wrote the manuscript, and had primary responsibility for the final content of the manuscript; ATW, KLT, and TJH: conducted the research; JMC, RZG, and AAF: conducted the research, analyzed data, and wrote the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: HFr, high fructose; HS, hepatic steatosis; LBP-1, LPS binding protein-1; MT, microbial translocation.
REFERENCES
- 1.Ford ES, Schulze MB, Bergmann MM, Thamer C, Joost HG, Boeing H. Liver enzymes and incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care 2008;31:1138–43. [DOI] [PubMed] [Google Scholar]
- 2.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA 2009;106:15430–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol 2008;49:600–7. [DOI] [PubMed] [Google Scholar]
- 5.Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N Engl J Med 2010;363:1341–50. [DOI] [PubMed] [Google Scholar]
- 6.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155–61. [DOI] [PubMed] [Google Scholar]
- 7.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 2011;140:124–31. [DOI] [PubMed] [Google Scholar]
- 8.Sievenpiper JL, de Souza RJ, Mirrahimi A, Yu ME, Carleton AJ, Beyene J, Chiavaroli L, Di Buono M, Jenkins AL, Leiter LA, et al. Effect of fructose on body weight in controlled feeding trials: a systematic review and meta-analysis. Ann Intern Med 2012;156:291–304. [DOI] [PubMed] [Google Scholar]
- 9.Coate KC, Scott M, Farmer B, Moore MC, Smith M, Roop J, Neal DW, Williams P, Cherrington AD. Chronic consumption of a high-fat/high-fructose diet renders the liver incapable of net hepatic glucose uptake. Am J Physiol Endocrinol Metab 2010;299:E887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohli R, Kirby M, Xanthakos SA, Softic S, Feldstein AE, Saxena V, Tang PH, Miles L, Miles MV, Balistreri WF, et al. High-fructose, medium chain trans fat diet induces liver fibrosis and elevates plasma coenzyme Q9 in a novel murine model of obesity and nonalcoholic steatohepatitis. Hepatology 2010;52:934–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M. Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 2008;22:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kavanagh K, Fairbanks LA, Bailey JN, Jorgensen MJ, Wilson M, Zhang L, Rudel LL, Wagner JD. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15:1666–74. [DOI] [PubMed] [Google Scholar]
- 13.Balzan S, de Almeida Quadros C, de Cleva R, Zilberstein B, Cecconello I. Bacterial translocation: overview of mechanisms and clinical impact. J Gastroenterol Hepatol 2007;22:464–71. [DOI] [PubMed] [Google Scholar]
- 14.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008;57:1470–81. [DOI] [PubMed] [Google Scholar]
- 15.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, Corthesy I, Mace K, Chou CJ. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. FASEB J 2008;22:2416–26. [DOI] [PubMed] [Google Scholar]
- 16.Riordan SM, McIver CJ, Williams R. Liver damage in human small intestinal bacterial overgrowth. Am J Gastroenterol 1998;93:234–7. [DOI] [PubMed] [Google Scholar]
- 17.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011;140:976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pappo I, Becovier H, Berry EM, Freund HR. Polymyxin B reduces cecal flora, TNF production and hepatic steatosis during total parenteral nutrition in the rat. J Surg Res 1991;51:106–12. [DOI] [PubMed] [Google Scholar]
- 19.Bergheim I, Weber S, Vos M, Kramer S, Volynets V, Kaserouni S, McClain CJ, Bischoff SC. Antibiotics protect against fructose-induced hepatic lipid accumulation in mice: role of endotoxin. J Hepatol 2008;48:983–92. [DOI] [PubMed] [Google Scholar]
- 20.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermudez-Humaran LG, Smirnova N, Berge M, Sulpice T, Lahtinen S, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med 2011;3:559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 2011;108(suppl 1):4586–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kavanagh K, Jones KL, Sawyer J, Kelley K, Carr JJ, Wagner JD, Rudel LL. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 2007;15:1675–84. [DOI] [PubMed] [Google Scholar]
- 23.Kavanagh K, Davis MA, Zhang L, Wilson MD, Register TC, Adams MR, Rudel LL, Wagner JD. Estrogen decreases atherosclerosis in part by reducing hepatic acyl-CoA:cholesterol acyltransferase 2 (ACAT2) in monkeys. Arterioscler Thromb Vasc Biol 2009;29:1471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bremer AA, Stanhope KL, Graham JL, Cummings BP, Wang W, Saville BR, Havel PJ. Fructose-fed rhesus monkeys: a nonhuman primate model of insulin resistance, metabolic syndrome, and type 2 diabetes. Clin Transl Sci 2011;4:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kritchevsky D, Davidson LM, Kim HK, Krendel DA, Malhotra S, Mendelsohn D, van der Watt JJ, duPlessis JP, Winter PA. Influence of type of carbohydrate on atherosclerosis in baboons fed semipurified diets plus 0.1% cholesterol. Am J Clin Nutr 1980;33:1869–87. [DOI] [PubMed] [Google Scholar]
- 26.Mubiru JN, Garcia-Forey M, Higgins PB, Hemmat P, Cavazos NE, Dick EJ, Jr, Owston MA, Bauer CA, Shade RE, Comuzzie AG, et al. A preliminary report on the feeding of cynomolgus monkeys (Macaca fascicularis) with a high-sugar high-fat diet for 33 weeks. J Med Primatol 2011;40:335–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem 2012;23:203–8. [DOI] [PubMed] [Google Scholar]
- 28.Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012;35:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high fructose corn syrup does not increase liver fat or ectopic fat in muscles. Appl Physiol Nutr Metab (in press). [DOI] [PubMed] [Google Scholar]
- 30.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr 1980;33:273–8. [DOI] [PubMed] [Google Scholar]
- 31.Hallfrisch J, Ellwood KC, Michaelis OEt, Reiser S, O'Dorisio TM, Prather ES. Effects of dietary fructose on plasma glucose and hormone responses in normal and hyperinsulinemic men. J Nutr 1983;113:1819–26. [DOI] [PubMed] [Google Scholar]
- 32.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marriott BP, Cole N, Lee E. National estimates of dietary fructose intake increased from 1977 to 2004 in the United States. J Nutr 2009;139:1228S–35S. [DOI] [PubMed] [Google Scholar]
- 34.Bremer AA, Auinger P, Byrd RS. Sugar-sweetened beverage intake trends in US adolescents and their association with insulin resistance-related parameters. J Nutr Metab 2010;2010:196476.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelmalek MF, Suzuki A, Guy C, Unalp-Arida A, Colvin R, Johnson RJ, Diehl AM. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010;51:1961–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spruss A, Kanuri G, Stahl C, Bischoff SC, Bergheim I. Metformin protects against the development of fructose-induced steatosis in mice: role of the intestinal barrier function. Lab Invest 2012;92:1020–32. [DOI] [PubMed] [Google Scholar]
- 37.Volynets V, Spruss A, Kanuri G, Wagnerberger S, Bischoff SC, Bergheim I. Protective effect of bile acids on the onset of fructose-induced hepatic steatosis in mice. J Lipid Res 2010;51:3414–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagnerberger S, Spruss A, Kanuri G, Volynets V, Stahl C, Bischoff SC, Bergheim I. Toll-like receptors 1-9 are elevated in livers with fructose-induced hepatic steatosis. Br J Nutr 2012;107:1727–38. [DOI] [PubMed] [Google Scholar]
- 39.Amar J, Burcelin R, Ruidavets JB, Cani PD, Fauvel J, Alessi MC, Chamontin B, Ferrieres J. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr 2008;87:1219–23. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Navarrete JM, Ortega F, Serino M, Luche E, Waget A, Pardo G, Salvador J, Ricart W, Fruhbeck G, Burcelin R, et al. Circulating lipopolysaccharide-binding protein (LBP) as a marker of obesity-related insulin resistance. Int J Obes (Lond) 2012;36:1442–9. [DOI] [PubMed] [Google Scholar]
- 41.Huang S, Rutkowsky JM, Snodgrass RG, Ono-Moore KD, Schneider DA, Newman JW, Adams SH, Hwang DH. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J Lipid Res 2012;53:2002–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trevisi P, De Filippi S, Minieri L, Mazzoni M, Modesto M, Biavati B, Bosi P. Effect of fructo-oligosaccharides and different doses of Bifidobacterium animalis in a weaning diet on bacterial translocation and Toll-like receptor gene expression in pigs. Nutrition 2008;24:1023–9. [DOI] [PubMed] [Google Scholar]
- 43.Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology 2009;50:1094–104. [DOI] [PubMed] [Google Scholar]
- 44.Basciano H, Miller AE, Naples M, Baker C, Kohen R, Xu E, Su Q, Allister EM, Wheeler MB, Adeli K. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am J Physiol Endocrinol Metab 2009;297:E462–73. [DOI] [PubMed] [Google Scholar]
- 45.Payne AN, Chassard C, Lacroix C. Gut microbial adaptation to dietary consumption of fructose, artificial sweeteners and sugar alcohols: implications for host-microbe interactions contributing to obesity. Obes Rev 2012;13:799–809. [DOI] [PubMed] [Google Scholar]
- 46.Claesson MJ, Jeffery IB, Conde S, Power SE, O'Connor EM, Cusack S, Harris HM, Coakley M, Lakshminarayanan B, O'Sullivan O, et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012;488:178–84. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.