Table 1.
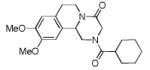
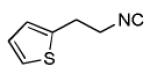
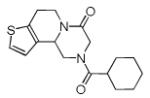
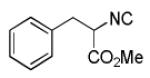
Varations of isocyanides and praziquantel derivatives 8(a-e)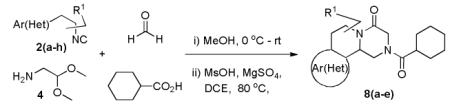
| Entry | R1NC 2(a-h) |
Product |
8 (a-e) |
Yield (%) |
|---|---|---|---|---|
| 1 |
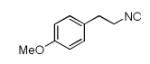
|
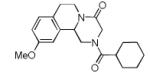
|
8a | 46c |
| 2 |
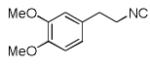
|
|
8b | 48c |
| 3 |
|
|
8c | 34c |
| 4 |
|
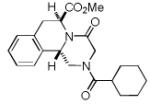
|
8d a | 16c |
| 5 |
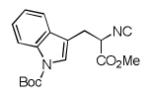
|
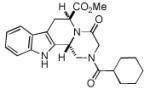
|
8e b | 45c |
| 6 |
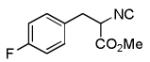
|
- | - | 76d |
| 7 |
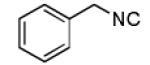
|
- | - | 73d |
| 8 |
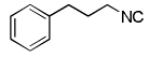
|
- | - | 58d |
Only trans-8d was identified.
Two diastereomers were isolated in a ratio of 2.4:1 (trans/cis).
Overall isolated yield for two steps.
Isolated yield for the Ugi reaction.
