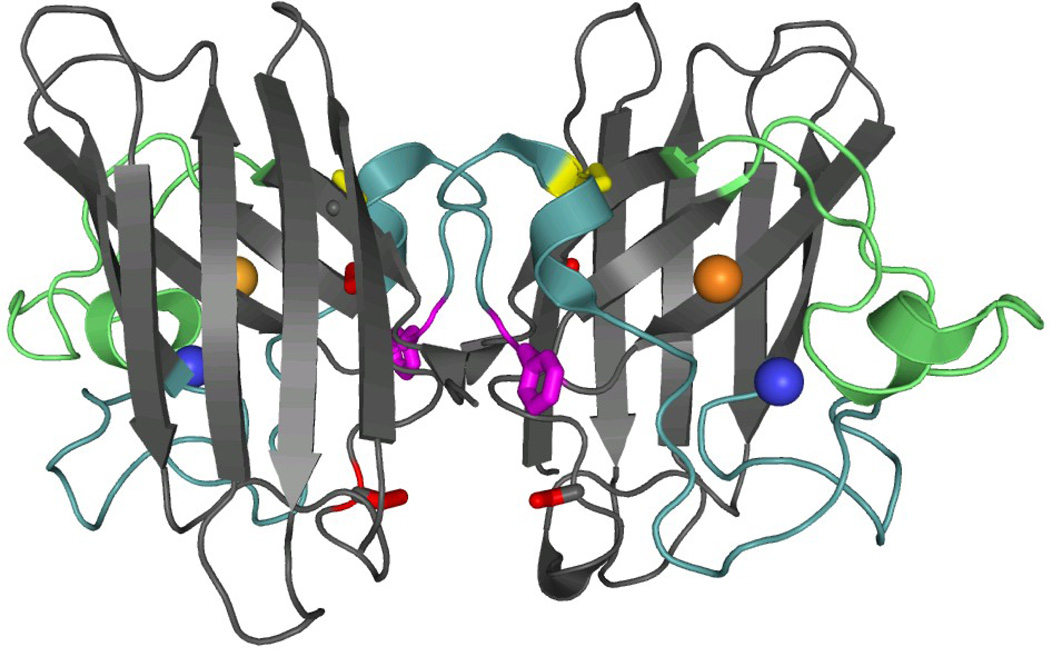
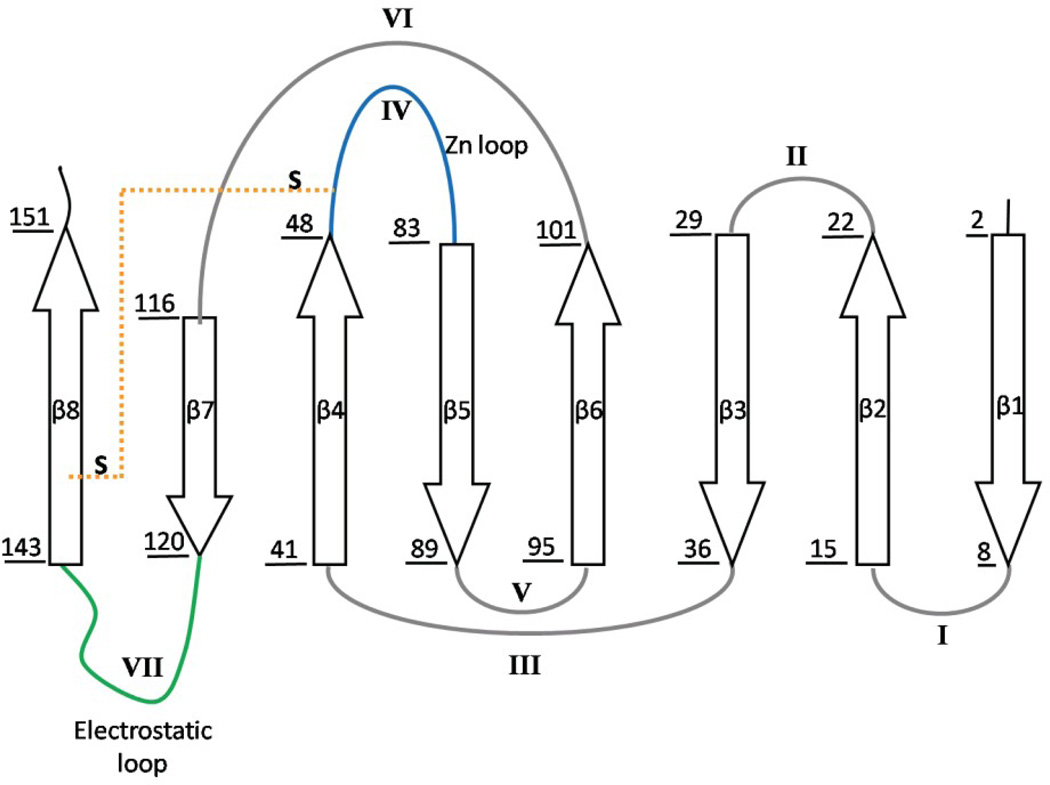
Figure 1. Crystal structure and topology of SOD1.
A) SOD1 is a dimeric β-sandwich protein consisting of eight anti-parallel β-stands supporting two catalytic loops (PDB: 2C9V). The electrostatic loop is depicted in green, while the Zn-binding loop is depicted in cyan. Each monomeric subunit also contains a Zn (blue sphere) and Cu ion (orange sphere), which were not present for this study, as well as an intramolecular disulfide bond (yellow). The free cysteines, C6/C111 (red), were replaced with alanine and serine respectively. Additionally, a pair of glutamic acid residues was introduced in the dimer interface, replacing F50/G51 (violet), in order to obtain the obligate monomer, mSOD1*. B) The topology of SOD1 has the immunoglobulin fold. It is made up of two β-sheetsconsisting of strands β1β2β3β6 and β5β4β7β8, and contains a Greek-key motif. The loops have been labeled with sequential Roman numerals.