Abstract
Objective
Little is known about the impact of hot flashes on cardiac autonomic regulation, in particular vagal control. Thereby, we assessed the cardiac autonomic profile associated with physiological hot flashes occurring in undisturbed sleep.
Methods
Eleven perimenopausal women (45 to 56 years) had overnight laboratory recordings of polysomnography, electrocardiography, and skin conductance. 18 hot flashes that occurred in stable non-rapid eye movement sleep undisturbed by arousals were analyzed. Heart rate variability measures were obtained for three consecutive 2-min windows starting from 4 min before (baseline and pre-flash periods) to 2 min after the onset of hot flashes (hot flash period).
Results
Heart rate increased by, on average, 4 beats per minute with the occurrence of a hot flash compared to both baseline (p < 0.001) and pre-flash (p < 0.001). High frequency power was reduced, reflecting a decrease in vagal activity, at the onset of a hot flash compared to baseline (p < 0.001) and pre-flash (p < 0.001). There was no change in sympathovagal balance with the onset of a hot flash. The magnitude of the hot flash, i.e. skin conductance amplitude, was associated with increased heart rate (r = 0.78, p < 0.001) and decreased vagal tone (r = -0.56, p = 0.014).
Conclusions
Physiological hot flashes per se, recorded during undisturbed sleep periods and independent of any arousals, are associated with increased heart rate and decreased cardiac autonomic vagal activity. These data support the hypothesis that the parasympathetic branch of the autonomic nervous system is involved in the cardiac response to a hot flash.
Keywords: Hot flash, sleep, autonomic activity, hot flush, heart rate variability, menopause
Introduction
Hot flashes are one of the most common and bothersome symptoms in menopause, and are reported by up to 84% of women in natural menopause. 1 They are subjectively portrayed as a sensation of heat, sweating, flushing, anxiety and chills and they typically last 1–5 min. 2 Several physiological changes accompany a subjective hot flash, including increased peripheral blood flow, skin temperature, sweating, and heart rate. 3-5 Skin conductance, the reciprocal of skin resistance, increases as sweat gland activity increases and is considered a valid objective indicator of a hot flash. 6-8 Hot flashes have a substantial impact on quality of life, interfering with work and daily activities as well as with sleep. 9 Hot flashes are strongly associated with difficulty sleeping10 and with chronic insomnia. 11
It is hypothesized that the withdrawal of estrogen that accompanies the approach to menopause affects hypothalamic alpha-2 adrenergic receptors and that the associated increase in brain norepinephrine levels narrows the thermoneutral zone in symptomatic women. 12 Consequently, relatively small increases in body temperature trigger a hot flash. 12 There is considerable support for the involvement of sympathetic activation in triggering hot flashes. Changes in skin resistance following excitation of sweat glands by sympathetic cholinergic fibers, and tachycardia at the onset of menopausal hot flashes, could be interpreted as an increase in sympathetic drive. 3, 5 Moreover, the norepinephrine metabolite, 3-methoxy-4-hydroxyphenylglycol is increased in plasma after a hot flash. 13 Yohimbine (an α2-adrenergic antagonist) has been found to provoke hot flashes while clonidine (an α2-adrenergic agonist) reduces them. 3 Recently, noninvasive indices considered to reflect relatively cardiac sympathetic dominance (low frequency component; LF: 0.03-0.15 Hz) and cardiac vagal dominance (high frequency component; HF: 0.15-0.40 Hz)14 derived from the heart rate variability (HRV) analysis of inter beats intervals (IBIs) have been investigated in association with the hot flash. 15-18 The time interval between adjacent heart beats (ms) is commonly known in its transformed form, i.e. the heart rate or myocardial contraction rate (beats per minute). The frequency spectral indices related to autonomic functioning (LF and HF) are usually computated using a Fast Fourier Transform which decomposes the variance of a time series into a frequency domain representation. 19 Whereas the HF activity is generally related to respiratory sinus arrhythmia (RSA), there is less consensus about the physiological meaning of the LF component: 19 although LF activity is largely considered a sympathetic index or a mixture of sympathetic and parasympathetic rhythms, 19 others studies support its relation with the baroreflex function.20, 21
To our knowledge, four studies have investigated changes in heart rate variability indices during hot flashes. Freedman et al. 18 found that power in the 0-0.15 Hz frequency band was elevated during a 5-min block at the onset of a nocturnal physiologic hot flash compared to a 5-min block after a hot flash, regardless of sleep or wake state, which the authors took to support the hypothesis of increased sympathetic activation as a trigger for hot flashes. Hoikkala and colleagues also reported an increase in very low and low frequency activity as well as a decrease in high frequency power during a 30-minute window encompassing a reported nocturnal hot flash compared to a preceding 30-min window in which hot flashes were not reported. 15 Thurston and colleagues found a decrease in high frequency power during a physiologic hot flash compared with either before or after a hot flash in women undergoing laboratory hot flash provocation testing with a stress task. 16 In a subsequent 24-h ambulatory study, high frequency power was also found to be decreased for either reported or physiologic hot flashes. 17 Low frequency power and the ratio of low frequency to high frequency power were also increased, suggesting that the decrease in high frequency power (vagal activity) predominated. 17 These studies provide mixed data as to whether the increase in heart rate apparent at the onset of a hot flash is due to reduced vagal activity and/or increased sympathetic activity.
Reasons for the discrepancies between studies may, in part, be due to differences in methodology, with hot flashes being triggered by a stressor or spontaneously occurring, being physiologically or subjectively assessed, recorded during different time periods (day and/or night), as well as differences in the definitions of hot flashes and HRV frequency bands. An important factor that has not been previously considered is whether the hot flash itself or other associated events, such as arousals, drive a change in cardiac autonomic regulation. The measurement of heart rate variability is highly sensitive to changes in sleep-wake state, 22, 23 arousals from sleep, 23 mental stress, 24 or emotional state. 25 Even brief arousals from sleep, lasting less than 30 s, are associated with substantial changes in autonomic regulation, with an increase in low frequency activity and decrease in high frequency activity, representing a shift to sympathetic dominance. 23 To isolate the association between hot flashes per se and autonomic regulation, it therefore is necessary to consider these factors. It is also necessary to adopt objective criteria to define clean windows for the analyses of heart rate variability.
To further investigate the autonomic profile in association with the onset of a hot flash, we used sleep as a model in which the flashes were free from disruptive events that occur during wake conditions. Further, we isolated ‘pure’ autonomic changes associated with a hot flash from sleep stage transitions and arousals from sleep, which are well known to impact the functioning of the autonomic system. 23, 26 Thus, we analyzed the autonomic activity associated with spontaneously-occurring hot flashes in stable non-rapid eye-movement sleep in a group of perimenopausal women.
Methods
Sample
Eleven perimenopausal women (Age: 50.2y ± 3.2y) who reported menopausal symptoms including vasomotor symptoms were recruited from the community. They completed a brief phone screen and if deemed eligible, were invited to the laboratory for a structured clinical interview. Inclusion criteria were: perimenopausal or early postmenopausal according to Stages of Reproductive Aging Workshop criteria, 27 i.e. menstrual cycle lengths that differed by more than 7 days from normal constituted early menopausal transition; an interval of amenorrhea of 60 or more days constituted late menopausal transition; amenorrhea for a period of up to 12 months (early postmenopausal). Other inclusion criteria were: report an average of at least one hot flash per day in the previous 2-weeks; body mass index (BMI, kg m-2) ≤ 32; intact uterus and at least one ovary; no use of hormone therapy (HT) or hormonal contraception for the previous 3 months; no hypertension or arrhythmias, no chronic medication use; and no current Axis I depressive disorder determined from structured clinical interview. All but one of the women were non-smokers and none of the women drank more than 7 alcoholic drinks per week. Three women reported no regular physical activity, four women reported moderate physical activity (e.g. Yoga or Pilates for about 1 hour/week), and four women reported heavy physical activity (e.g. Running for about 1 hour/week).
This study followed the Declaration of Helsinki on medical protocol and ethics and was approved by the Institutional Review Board at SRI International. All participants read and signed the informed consent.
Procedure
Participants underwent polysomnographic (PSG) recordings at the Human Sleep Research Laboratory at SRI International. They arrived at the laboratory around 7 pm and sensors were attached. Participants slept in sound-attenuated and temperature-controlled bedrooms. Lights-out and lights-on times were self-selected by participants.
Polysomnographic assessment
Electroencephalographic (EEG; C3-A2, C4-A1, O1-A2, O2-A1), electrooculographic (EOG), and submental electromyographic (EMG) recordings were made using E-series amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia) linked to appropriate transducers. EEG signals were digitized at a sampling rate of 256 Hz, high-pass filtered at 0.3 Hz, and low-pass filtered at 30 Hz. Thirty-second epochs were scored (wake, N1, N2, N3 and rapid eye movement (REM)) by experienced scorers following the American Academy of Sleep Medicine (AASM) rules. 28 Arousals were scored using the AASM criteria; 28 they were defined by an increase in fast EEG activity relative to background activity that lasted at least 3 s.
Cardiac autonomic assessment
Electrocardiographic (ECG) recordings were also assessed with E-series amplifiers and Profusion software (Compumedics, Abbotsford, Victoria, Australia) using Ag/AgCl Meditrace surface spot electrodes in a modified Lead II Einthoven configuration. Data were continuously collected at 512 Hz. Power spectral heart rate variability analysis (HRV) was performed using dedicated software developed at the University of Melbourne and according to procedures previously described. 29 Briefly, R waves were automatically detected by the software, visually checked and manually adjusted when necessary. Inter-beat-intervals were re-sampled at 4 Hz and filtered with a third-order polynomial filter to remove very low frequency components. Power spectrum analysis was applied to the time series. The resulting frequency bands were 0.02 Hz wide over the range from 0 to 0.5 Hz. To identify the low frequency (LF) component the peak frequency band between 0.03 and 0.15 Hz was identified. The width of the LF component was then defined by the first frequency bands either side of the peak to fall to 50% of the peak value. The area so defined was then integrated. The same procedure was used to identify the HF component between 0.15 and 0.40 Hz. We derived the following measures: heart rate (HR; bpm); absolute power in the low frequency band (LFa, arbitrary units), an indicator of vagal and sympathetic nervous system activity combined; 19 absolute power in the high frequency band (HFa, arbitrary units), an indicator of vagal activity; ratio of low-to-high frequency (LFa/HFa), an indicator of sympathovagal balance; peak frequency in the high frequency range (HFp, Hz), a measure of respiratory rate. 29
Hot flash recording and characterization
Physiological hot flashes were assessed based on measures of sternal skin conductance (SC) with a BioDerm Skin Conductance Meter (Model 2701, UFI, Morro Bay, California, USA) which maintained a 0.5 V constant voltage circuit30 while sampling from two silver/silver chloride electrodes placed on either side of the sternum. The Bioderm was connected via an optically isolated DC-input to the Compumedics amplifiers and sampled at 16 Hz. Electrodes were 1.5 cm in diameter and filled with 0.05 M potassium chloride Velvachol/glycol gel. 31 SC recordings were manually evaluated by two scorers (FCB and MdZ) for fluctuations meeting accepted criteria for physiological hot flashes (an increase of ≥ 2 micromhos within 30 seconds). 8 The beginning of this 30-s epoch of increased SC was used to define the onset of a hot flash.
Only hot flashes meeting these criteria and that had an onset during stable NREM sleep (N2 and N3 sleep stages) were selected for analysis. In addition, the four minutes before and the two minutes after the onset of the hot flash were free from body movement, micro-arousals, awakenings and transitions to N1 or REM sleep (Fig. 1).
FIG. 1.
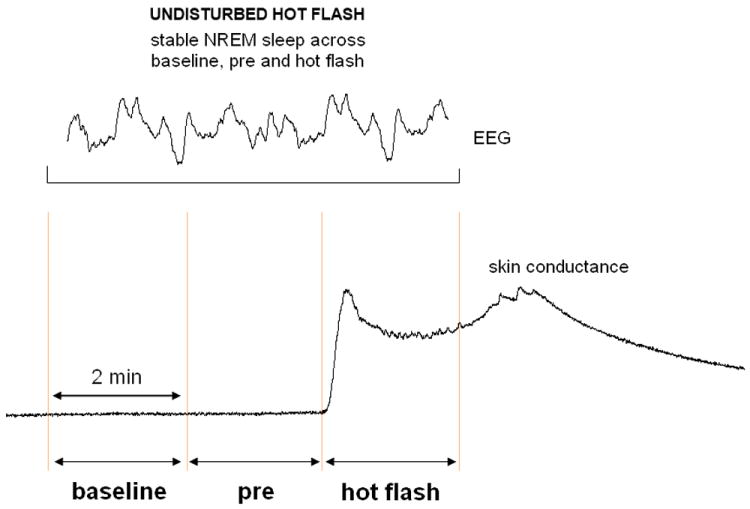
Graphical representation of how hot flashes are defined. Three 2-minute windows (baseline, preflash, and hot flash) were selected for analysis, from 4 minutes before the onset of a hot flash to 2 minutes after the onset of a hot flash (marked by an abrupt increase in skin conductance). Pure hot flashes occurred in stable non–rapid eye movement (NREM) sleep (an electroencephalographic [EEG] segment shows the high-amplitude and low-frequency activity characterizing the period). Hot flashes were not included in the analysis if they occurred during arousal (characterized by fast EEG activity for at least 3 s with or without an increase in muscle tone), if they occurred during sleep stage change, or if an awakening occurred.
A total of 18 hot flashes met the above criteria and constituted the final sample. Hot flashes that occurred during wakefulness (n = 8), during REM sleep (n = 4) and those that occurred across sleep stage transitions and/or were associated with arousals/awakenings (n = 28) were not analyzed due to the unstable period of recording and/or to the association of hot flashes with other disruptive events such as arousals.
Data reduction and analysis
Two minute epochs were selected, before (pre-flash; -2 to 0 min) and at the onset (hot flash; 0 to 2 min) of each hot flash period. An additional 2-min artifact free baseline (-4 to -2 min) period occurring before the pre-flash period was also selected for analysis (Fig. 1).
The SC was normalized before data analysis to reduce the between subject variability. Normalized amplitude (ranging from 0 to 1 μmho) of the SC signal (SCamp; μmhos) was calculated. The HRV measures, LFa, HFa and LFa/HFa were log transformed before statistical analysis. SCamp and HR variables were averaged over each 2 minute epoch.
All variables (SCamp, HR, LFa, HFa, HFp and LFa/HFa) were analyzed with hierarchical mixed effect models with a fixed effect for pre-flash and hot flash, so that the constant represented the baseline. Random effects accounting for between subjects (subject-to-subject) and within subjects (flash-to-flash) variability (part of the total variation seen across all hot flashes) were included since women contributed different numbers of hot flashes to the analysis. The chi square (χ2) value is reported for each model. In addition, z values are reported for each hot flash period. Estimated variance and 95% confident intervals of the random effects parameters and the residual variance are provided for each model. All analyses were performed using Stata/SE 12.1 for Windows.
To analyze the relation between the magnitude of hot flashes and the associated autonomic changes Pearson’s correlations were calculated between differential values of SCamp and HR, LFa, HFa and LFa/HFa. Differential values were calculated as the mean of the absolute values in the hot flash period minus the mean of the absolute values in the baseline period. Pearson product-moment correlation coefficients (r) and linear equations are reported for the significant correlations.
A probability level p < 0.05 was set for significance.
Results
Autonomic modifications during hot flashes
Means and standard deviations for all physiological measures recorded during the 2 minute baseline, pre-flash and hot flash conditions, as well as hierarchical mixed effect model results are provided in Table 1. Estimated variance and 95% confident intervals of the random effects parameters and the residual variance are provided in Table 2.
Table 1.
Physiological variables (mean ± SD) recorded during baseline, pre and hot flash periods and hierarchical mixed effect model results. Data are based on a sample of 18 hot flashes recorded from 11 women.
| Baseline | Pre | Hot Flash | χ2 | p | |
|---|---|---|---|---|---|
| SCamp (μohm) | 0.28 ± 0.14 | 0.28 ± 0.14 | 0.72 ± 0.18 | 286.79 | < 0.001 |
| HR (bpm) | 61.34 ± 10.99 | 62.69 ± 11.16 | 65.28 ± 12.09 | 71.49 | < 0.001 |
| LFa (log) | 1.22 ± 0.47 | 1.23 ± 0.37 | 1.04 ± 0.33 | 4.52 | 0.104 |
| HFa (log) | 1.79 ± 0.46 | 1.72 ± 0.47 | 1.55 ± 0.54 | 22.35 | < 0.001 |
| HFp (Hz) | 0.23 ± 0.03 | 0.23 ± 0.03 | 0.24 ± 0.03 | 7.49 | 0.024 |
| LFa/HFa (log) | -0.57 ± 0.60 | -0.49 ± 0.54 | -0.51 ± 0.57 | 0.59 | 0.745 |
HF, high frequency (a = absolute power in narrow band; p = peak frequency); HR, heart rate; LF, low frequency (a = absolute power in narrow band); SC, skin conductance (amp = amplitude).
Table 2.
Estimated variance and 95% confident intervals of the random effects parameters and the residual variance for each model. Data are based on a sample of 18 hot flashes recorded from 11 women.
| Random-Effects | Variance estimate | 95% Confident Intervals | ||
|---|---|---|---|---|
| SCamp (μohm) | Between subjects | 0.07 | 0.02 | 0.29 |
| Within subjects | 0.11 | 0.06 | 0.19 | |
| Residual | 0.09 | 0.07 | 0.11 | |
| HR (bpm) | Between subjects | 10.10 | 6.18 | 16.50 |
| Within subjects | 3.44 | 2.07 | 5.71 | |
| Residual | 1.42 | 1.12 | 1.80 | |
| LFa (log) | Between subjects | 0.17 | 0.01 | 4.30 |
| Within subjects | 0.19 | 0.03 | 1.07 | |
| Residual | 0.31 | 0.24 | 0.39 | |
| HFa (log) | Between subjects | 0.23 | 0.04 | 1.17 |
| Within subjects | 0.41 | 0.25 | 0.67 | |
| Residual | 0.16 | 0.12 | 0.20 | |
| HFp (Hz) | Between subjects | 0.030 | 0.017 | 0.053 |
| Within subjects | 0.012 | 0.006 | 0.025 | |
| Residual | 0.012 | 0.010 | 0.016 | |
| LFa/HFa (log) | Between subjects | 0.35 | 0.12 | 1.03 |
| Within subjects | 0.35 | 0.18 | 0.70 | |
| Residual | 0.31 | 0.25 | 0.40 | |
HF, high frequency (a = absolute power in narrow band; p = peak frequency); HR, heart rate; LF, low frequency (a = absolute power in narrow band); SC, skin conductance (amp = amplitude).
As per their definition, hot flashes were characterized by abrupt increases in SCamp during hot flash compared to baseline (z = 14.60, p < 0.001) and pre-flash (z = 14.73, p < 0.001) conditions. No differences were detected between baseline and pre-flash.
Heart rate significantly accelerated in hot flash compared with both baseline (z = 8.32, p < 0.001) and pre-flash (z = 5.46, p < 0.001) conditions. A smaller but significant increase in HR was detected during pre-flash compared to baseline (z = 2.86, p = 0.004) (Fig. 2).
FIG. 2.
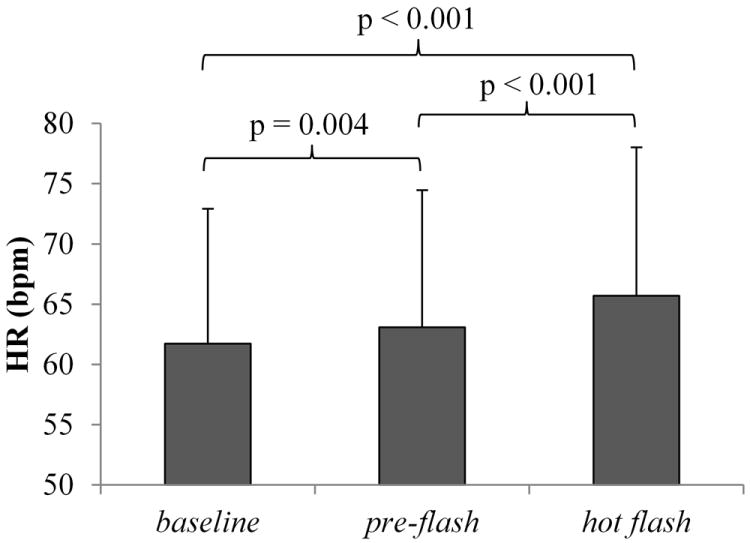
Mean (SD) heart rate (HR) across hot flash periods in undisturbed hot flashes. HR was increased in the preflash and hot flash periods compared with baseline, as well as in the hot flash period compared with preflash. Data represent a sample of 18 hot flashes recorded from 11 women.
HFa, a vagal index, decreased from baseline to the hot flash (z = -4.60, p < 0.001) as well as from pre to the hot flash (z = -3.26, p = 0.001) (Fig. 3). HFp, an index considered reflecting the respiratory rate, slightly increased from baseline to the hot flash (z = 2.63, p = 0.009) as well as from pre to the hot flash (z = 1.97, p = 0.049)
FIG. 3.
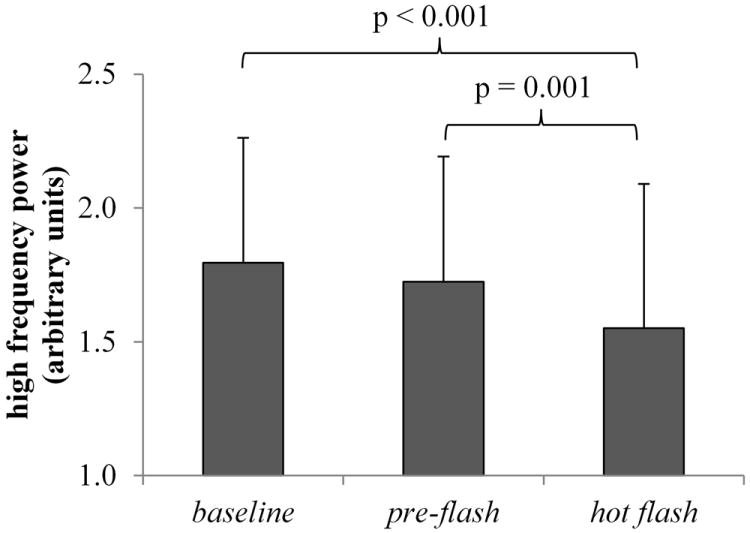
High-frequency power (reflecting vagal activity) derived from heart rate variability analysis across hot flash periods in undisturbed hot flashes. Vagal activity was reduced in the hot flash period relative to preflash and baseline. Values are reported in logarithms of arbitrary units. Data represent a sample of 18 hot flashes recorded from 11 women.
LFa and LFa/HFa ratio did not show any significant modification in association with a hot flash.
Relationship between hot flash magnitude and associated autonomic responses
Pearson’s correlations revealed significant correlations between increases in SCamp and changes in autonomic activity from baseline-to-hot flash. ΔSCamp was positively correlated with ΔHR (r = 0.78, p < 0.001) (Fig. 4a) and was negatively correlated with ΔHFa (r = -0.56, p = 0.014) (Fig. 4b).
FIG. 4.
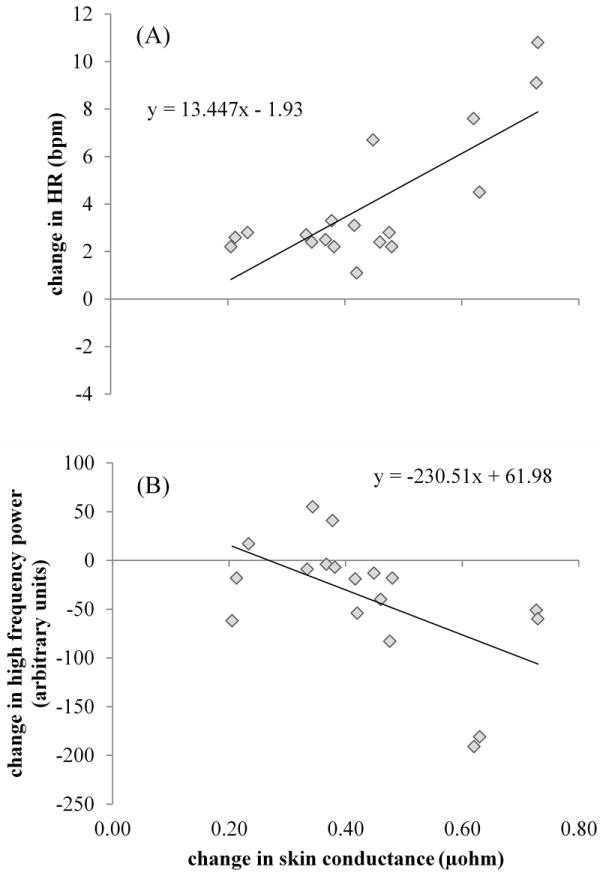
A: Positive correlation between change score values (calculated as hot flash minus baseline absolute values) of heart rate (HR) and skin conductance. B: Negative correlation between increases in skin conductance and reductions in high-frequency power (ie, reduction in vagal outflow). Data represent a sample of 18 hot flashes recorded from 11 women.
Discussion
To our knowledge, this is the first study to show the independent effect of hot flashes on cardiac autonomic activity. By using strict criteria to characterize hot flashes and analyze heart rate variability, we identified components of the cardiac autonomic pattern that accompany the onset of a hot flash during undisturbed NREM sleep.
Hot flashes were associated with a small but significant increase in HR at the onset of a hot flash compared to the periods preceding it (~4 bpm), together with a reduction in vagal activity (reduction of about 38% in HF-HRV power). This finding suggests that the increase in HR at the onset of a hot flash is due primarily to a withdrawal of vagal activation and not due to an increase in sympathetic activity. The correlation analysis supports a relationship between the magnitude of hot flashes (increase in skin conductance amplitude) and autonomic fluctuations (increases in HR and decreases in HF power). Larger hot flashes, as indicated by larger skin conductance responses, therefore have a bigger impact on cardiac autonomic regulation.
Our finding of a reduction in vagal activity in association with hot flashes supports those of Thurston and colleagues. 16, 17 Our study extends those findings to show the effect of hot flashes independent of other potential disruptive events, such as arousals from sleep, on vagal activity. Our findings also show that physiologic hot flashes alone are not associated with a change in LF power, which is in contrast to previous findings. 17, 18 It is likely that the increase in LF power reported by those studies was due to the confounding effect of events such as arousals and shifts to Stage 1 or REM sleep, which lead to a shift in sympathetic dominance. 23
Research suggests an association between hot flashes and cardiovascular risk32 and a reduction in cardiac vagal control and increased cardiac sympathetic activation are associated with cardiovascular morbidity. 33 A decrease in high frequency power in association with hot flashes, as shown here, could be one potential mechanism linking vasomotor symptoms and cardiovascular risk. As Thurston and colleagues17 suggest, the role of the autonomic nervous system in mediating the link between hot flashes and cardiovascular risk should be considered further.
Hot flashes are hypothesized to be mediated through the sympathetic branch of the autonomic nervous system. 3, 5 Hot flashes are a strong heat dissipation response3 and the increase in skin conductance (i.e. reduction in skin resistance) is a peripheral index of sweat gland activity mediated by the sympathetic branch of the autonomic nervous system, acting on cholinergic receptors. 34 Along with an increase in sweating during a hot flash, there is also an increase in blood flow (i.e. vasodilatation), 4, 5, 35, 36 which likely is mediated by a reduction of sympathetic vasoconstrictor activity and/or by increases in sympathetic cholinergic vasodilator activity on the vasculature; 35 the thermoregulatory reflex of cutaneous vascular control during heat stress as well as cold stress in humans is mediated by the two branches of the sympathetic nervous system: the noradrenergic vasoconstrictor nerves and the cholinergic active vasodilator nerves. 37 Although the sympathetic nervous system branch may be involved in the peripheral heat loss response of a hot flash, our results suggest that it is the parasympathetic (vagal) branch that is involved in the cardiac response to a hot flash. Given the vagal dominance of the myocardium under resting states, 19 it is less likely that the cardiac response to hot flashes during sleep is due to sympathetic influence. Whether the increase in HR is a consequence of the vasodilatation in the periphery or coincident with it, remains to be confirmed. Further research should be focused on investigating autonomic measures around the onset of the skin conductance increases with a better time resolution to better understand the physiology of hot flashes. In addition, further studies should be focused on understanding the sympathetic input on myocardium using indices like the pre-ejection period, a noninvasive measure assessed by impedance cardiography and reflecting cardiac sympathetic beta-adrenergic activity. 38
Our findings should be considered within the context of the study limitations. Although HF power is accepted as a measure of vagal activity, 39 variations in respiratory activity can modulate this component. 29, 40 We therefore analyzed the frequency of the peak of the HF component (HFp) as a measure of respiratory rate. There was a small but significant increase in breathing frequency at the onset of a hot flash. However, given this small increase in respiratory rate (average peak HF value increase of less than 5%), it is unlikely that the decrease in vagal activity during a hot flash is driven by a change in respiration. The sample size was small and limited to perimenopausal women with a range in severity of vasomotor symptoms. The restricted sample of woman was largely due to the fact that in most of the cases hot flashes were followed by arousals, awakenings or sleep stage transition. However, in order to provide insight into the physiology of hot flashes, we carefully selected ‘pure’ hot flashes during sleep and show a robust effect of hot flashes on vagal regulation.
Conclusion
We have shown, for the first time, the clear impact of physiologic hot flashes on cardiac autonomic regulation. Hot flashes occurring during undisturbed sleep are independently associated with a reduction in vagal activity and an increase in heart rate. These data show that the parasympathetic branch of the autonomic nervous system is involved in the cardiac response to a hot flash.
Acknowledgments
We would like to thank Justin Greco, Rebecca Carr, David Sugarbaker and Ben Mayer for their effort in the data collection process, Weiwei Chu for assistance with data analysis, and Dr Harold Javitz for statistical expertise. This study was supported by National Institutes of Health, Bethesda, MD, USA; Grant HL103688 to F. C. B.
Funding: National Institutes of Health (NIH), NIH HL103688
Footnotes
Financial disclosure/conflicts of interest: Dr. Colrain reports consultancy with Breathe Technologies and grants/grants pending with Apnicure, Inc. No other conflicts reported.
References
- 1.Melby MK, Anderson D, Sievert LL, Obermeyer CM. Methods used in cross-cultural comparisons of vasomotor symptoms and their determinants. Maturitas. 2011;70(2):110–9. doi: 10.1016/j.maturitas.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Kronenberg F. Hot flashes: epidemiology and physiology. Ann N Y Acad Sci. 1990;592:52–86. doi: 10.1111/j.1749-6632.1990.tb30316.x. [DOI] [PubMed] [Google Scholar]
- 3.Freedman R. Physiology of hot flashes. Am J Hum Biol. 2001;13(4):453–64. doi: 10.1002/ajhb.1077. [DOI] [PubMed] [Google Scholar]
- 4.Kronenberg F, Cote L, Linkie D, Dyrenfurth I, Downey J. Menopausal hot flashes: thermoregulatory, cardiovascular, and circulating catecholamine and LH changes. Maturitas. 1984;6(1):31–43. doi: 10.1016/0378-5122(84)90063-x. [DOI] [PubMed] [Google Scholar]
- 5.Sturdee D, Wilson K, Pipili E, Crocker A. Physiological aspects of menopausal hot flush. Br Med J. 1978;2(6130):79–80. doi: 10.1136/bmj.2.6130.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter J, Azzouz F, Monahan P, Storniolo A, Ridner S. Is sternal skin conductance monitoring a valid measure of hot flash intensity or distress? Menopause. 2005;12(5):512–9. doi: 10.1097/01.gme.0000170957.31542.1c. [DOI] [PubMed] [Google Scholar]
- 7.Tataryn I, Lomax P, Bajorek J, Chesarek W, Meldrum D, Judd H. Postmenopausal hot flushes: a disorder of thermoregulation. Maturitas. 1980;2(2):101–7. doi: 10.1016/0378-5122(80)90043-2. [DOI] [PubMed] [Google Scholar]
- 8.Freedman R. Laboratory and ambulatory monitoring of menopausal hot flashes. Psychophysiology. 1989;26(5):573–9. doi: 10.1111/j.1469-8986.1989.tb00712.x. [DOI] [PubMed] [Google Scholar]
- 9.Sturdee DW. The menopausal hot flush—Anything new? Maturitas. 2008;60(1):42–9. doi: 10.1016/j.maturitas.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Kravitz H, Ganz P, Bromberger J, Powell L, Sutton-Tyrrell K, Meyer P. Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause. 2003;10(1):19–28. doi: 10.1097/00042192-200310010-00005. [DOI] [PubMed] [Google Scholar]
- 11.Ohayon M. Severe hot flashes are associated with chronic insomnia. Arch Intern Med. 2006;166(12):1262–8. doi: 10.1001/archinte.166.12.1262. [DOI] [PubMed] [Google Scholar]
- 12.Freedman R. Menopausal Hot Flashes. In: Lobo R, editor. Treatment of the postmenopausal woman. 3. New York: Academic Press; 2007. pp. 187–98. [Google Scholar]
- 13.Freedman R. Biochemical, metabolic, and vascular mechanisms in menopausal hot flashes. Fertil Steril. 1998;70(2):332–7. doi: 10.1016/s0015-0282(98)00137-x. [DOI] [PubMed] [Google Scholar]
- 14.Cacioppo J, Tassinary L, Berntson G. Handbook of psychophysiology. 3. New York, NY: Cambridge University Press; 2007. [Google Scholar]
- 15.Hoikkala H, Haapalahti P, Viitasalo M, et al. Association between vasomotor hot flashes and heart rate variability in recently postmenopausal women. Menopause. 2010;17(2):315–20. doi: 10.1097/gme.0b013e3181c2bb6d. [DOI] [PubMed] [Google Scholar]
- 16.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control: a link to cardiovascular risk? Menopause. 2010;17(3):456–61. doi: 10.1097/gme.0b013e3181c7dea7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurston R, Christie I, Matthews K. Hot flashes and cardiac vagal control during women’s daily lives. Menopause (New York, NY) 2012;19(4):406–12. doi: 10.1097/gme.0b013e3182337166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freedman R, Kruger M, Wasson S. Heart rate variability in menopausal hot flashes during sleep. Menopause (New York, NY) 2011;18(8):897–900. doi: 10.1097/gme.0b013e31820ac941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. European heart journal. 1996;17(3):354–81. [PubMed] [Google Scholar]
- 20.Moak J, Goldstein D, Eldadah B, et al. Supine low-frequency power of heart rate variability reflects baroreflex function, not cardiac sympathetic innervation. Heart rhythm: the official journal of the Heart Rhythm Society. 2007;4(12):1523–9. doi: 10.1016/j.hrthm.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sleight P, La Rovere M, Mortara A, et al. Physiology and pathophysiology of heart rate and blood pressure variability in humans: is power spectral analysis largely an index of baroreflex gain? Clin Sci (Lond) 1995;88(1):103–9. doi: 10.1042/cs0880103. [DOI] [PubMed] [Google Scholar]
- 22.Trinder J. Cardiac activity and sympathovagal balance during sleep. Sleep Med Clin. 2007;2(2):199–208. [Google Scholar]
- 23.Bonnet M, Arand D. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102(5):390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- 24.Hjortskov N, Rissén D, Blangsted A, Fallentin N, Lundberg U, Søgaard K. The effect of mental stress on heart rate variability and blood pressure during computer work. Eur J Appl Physiol. 2004;92(1-2):84–9. doi: 10.1007/s00421-004-1055-z. [DOI] [PubMed] [Google Scholar]
- 25.Appelhans BM, Luecken LJ. Heart Rate Variability as an Index of Regulated Emotional Responding. Rev Gen Psychol. 2006;10(3):229–40. [Google Scholar]
- 26.Trinder J, Waloszek J, Woods M, Jordan A. Sleep and cardiovascular regulation. Pflugers Arch. 2012;463(1):161–8. doi: 10.1007/s00424-011-1041-3. [DOI] [PubMed] [Google Scholar]
- 27.Soules M, Sherman S, Parrott E, et al. Stages of Reproductive Aging Workshop (STRAW) Journal of women’s health & gender-based medicine. 2001;10(9):843–8. doi: 10.1089/152460901753285732. [DOI] [PubMed] [Google Scholar]
- 28.Iber C. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. American Academy of Sleep Medicine. 2007 [Google Scholar]
- 29.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10(4):253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 30.Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology. 1971;8(5):656–72. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 31.Dormire S, Carpenter J. An alternative to Unibase/glycol as an effective nonhydrating electrolyte medium for the measurement of electrodermal activity. Psychophysiology. 2002;39(4):423–6. doi: 10.1017.S0048577201393149. [DOI] [PubMed] [Google Scholar]
- 32.Thurston RC, Sutton-Tyrrell K, Everson-Rose SA, Hess R, Matthews KA. Hot Flashes and Subclinical Cardiovascular Disease Findings From the Study of Women’s Health Across the Nation Heart Study. Circulation. 2008;118(12):1234–40. doi: 10.1161/CIRCULATIONAHA.108.776823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010;141(2):122–31. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- 34.Guyton A, Hall J. Textbook of medical physiology. 11. Philadelphia, PA: Saunders Elsevier; 2006. [Google Scholar]
- 35.Low DA, Davis SL, Keller DM, Shibasaki M, Crandall CG. Cutaneous and hemodynamic responses during hot flashes in symptomatic postmenopausal women. Menopause. 2008;15(2):290–5. doi: 10.1097/gme.0b013e3180ca7cfa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ginsburg J, Swinhoe J, O’Reilly B. Cardiovascular responses during the menopausal hot flush. Br J Obstet Gynaecol. 1981;88(9):925–30. doi: 10.1111/j.1471-0528.1981.tb02230.x. [DOI] [PubMed] [Google Scholar]
- 37.Kellogg D., Jr In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J Appl Physiol. 2006;100(5):1709–18. doi: 10.1152/japplphysiol.01071.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood A, Allen M, Fahrenberg J, Kelsey R, Lovallo W, van Doornen L. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27(1):1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- 39.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043–65. [PubMed] [Google Scholar]
- 40.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human RR interval power spectra is largely ignored. J Appl Physiol. 1993;75(5):2310–7. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]


