Abstract
Background
Dilated cardiomyopathy (DCM) is characterized by deteriorating cardiac performance and impaired contraction and dilation of the left (or both) ventricles. Blood markers – known as “biomarkers” allow insight into underlying pathophysiologic mechanisms and biologic pathways, while predicting outcomes and guiding heart failure management and/or therapies.
Content
In this review, we provide an alternative approach to conceptualize heart failure biomarkers: the cardiomyocyte, its surrounding microenvironment, and the macroenvironment with clear interaction between these entities which may impact cellular processes involved in the pathogenesis and/or propagation of DCM. Newer biomarkers of left ventricular systolic dysfunction can be categorized under: (a) myocyte stress and stretch, (b) myocyte apoptosis, (c) cardiac interstitium, (d) inflammation, (e) oxidative stress, (f) cardiac energetics, (g) neurohormones and (h) renal biomarkers.
Summary
Biomarkers provide insight into the pathogenesis of DCM while predicting and potentially providing prognostic information in these patients with heart failure.
Keywords: Biomarkers, dilated cardiomyopathy, heart failure
Cardiomyopathy is defined as an alteration in the structure and function of the myocardium, leading to deterioration of myocardial performance often resulting in the development of clinical heart failure. Dilated cardiomyopathy (DCM), a common cardiomyopathy leading to heart failure, has a prevalence of 1:2500 [1] and is characterized by enlargement of one or both of the ventricles with associated systolic dysfunction. Many diverse etiologies, either primary (solely or predominantly confined to the heart muscle) or secondary (myocardial involvement from a systemic disease process), may lead to the DCM phenotype. A diagnosis of DCM requires evidence of dilation and impaired contraction of the left ventricle or both ventricles (e.g., left ventricular ejection fraction (LVEF) < 40 percent) [2]. The disease is considered idiopathic if primary and secondary causes of heart disease are excluded. Of note, in the literature, the term DCM is usually an all-encompassing term for a non-ischemic cardiomyopathy with depressed left ventricular (LV) function.
Macroscopically, DCM consists of hearts that are heavy (increased LV mass) with geometric changes indicating eccentricity (defined as low relative wall thickness - normal or reduced wall thickness in relation to a dilated LV chamber size) [3]. Microscopically, cardiomyocytes in DCM often consist of a classic histological triad – myocyte hypertrophy, myocyte loss, and interstitial fibrosis [4]. However, these findings are imprecise in identifying underlying etiologies for cardiomyopathies. In addition, the natural course of DCM can be variable, contingent on multiple factors, including etiology and initial cardiac phenotype of the cardiomyopathy, on-going cardiac insults and genetic underpinnings of an individual’s resistance to adverse cardiac remodeling.
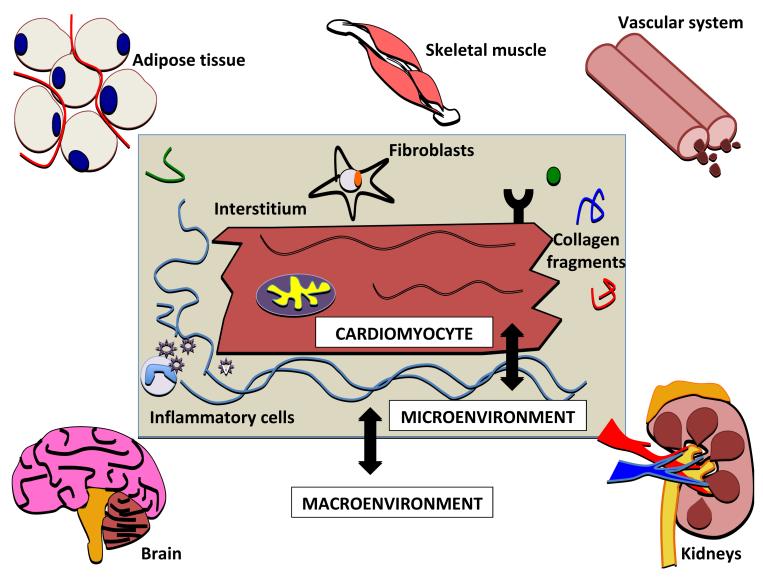
Heart failure is a clinical syndrome that manifests as a consequence of the progression of the underlying cardiomyopathy. It is a complex process and features pressure and/or volume overload leading and ventricular remodeling. Diverse etiologies, presentations, and outcomes are seen thus, making the classification of cardiomyopathies challenging. Equally challenging is categorizing biomarkers of heart failure as they are often described according to their mechanism (e.g., neurohormonal, oxidative stress etc.). However, an alternative method to conceptualize biomarkers in heart failure is to approach the classification from three vantage points: the cardiomyocyte, its surrounding microenvironment, and the macroenvironment (Figure 1). In heart failure, the cardiac myocyte is subjected to many stressors – such as mechanical, oxidative and pro-inflammatory, resulting in structural and functional changes (i.e., hypertrophy, necrosis/ apoptosis, altered myocyte energetics, impaired contraction and relaxation). The myocyte “microenvironment”, the environment immediately surrounding a myocyte, includes the cardiac interstitium, cardiac fibroblasts, and other factors that interact or cross-talk with cardiomyocytes. Lastly, the “macroenvironment” in heart failure refers to the interaction of the heart and other organ systems and the impact of those systems on the heart (e.g., insulin resistance, cardiac cachexia, obesity, cardiorenal syndrome and ventricular-vascular coupling). The intimate relationship between the micro- and macroenvironment with the cardiomyocyte may result in downstream cellular or signaling changes which may be important in the initiation and propagation of DCM or reflect changes that have already taken place in DCM.
Fig 1. Interaction of heart failure biomarkers between the cardiomyocyte, the microenvironment, and the macroenvironment.
Stressors on the cardiac myocyte lead to structural and functional changes (hypertrophy, apoptosis, altered myocyte energetics, and contraction apparatus modifications). Microenvironment refers to the immediate environment of the myocyte – the interstitium, cardiac fibroblasts, and inflammatory mediators. Lastly, the macroenvironment includes other organ systems and their impact on the heart in heart failure (e.g. adipose tissue, cardiac cachexia, cardiorenal syndrome, neurohormones, and ventricular-vascular coupling). Clear overlap exists between these domains contributing to the development and propagation of heart failure.
For the purpose of this review, emerging biomarkers in DCM will be the primary focus. Although 20-35% of DCM has been genetically correlated with greater than 20 loci and associated genes [1], genetic biomarkers are out of the scope of this review and will not be discussed. The biomarkers reviewed are not unique to DCM, and we do not provide an exhaustive list (Table 1) but provide insight into underlying pathophysiologic mechanisms and biologic pathways important in, but not specific to DCM. Some of these biomarkers may prognosticate outcomes, enable guidance in heart failure therapeutics and may be used for monitoring treatments in DCM.
Table 1.
Biomarkers in Dilated Cardiomyopathy (DCM)
|
CARDIOMYOCYTE -Brain natriuretic peptide* -N-terminal brain natriuretic peptide* -Atrial natriuretic peptide* -Soluble ST2* -Heart-type fatty acid binding protein* -Soluble apoptosis stimulating fragment* -Myostatin |
MICROENVIRONMENT -Matrix metalloproteinases* -Tissue inhibitors of metalloproteinases* -Collagen propeptides* -Galectin-3* -Tumor necrosis factor-like weak inhibitory of apoptosis -Osteoprotegerin* -Pentraxin-3* -Cardiotrophin-1 -Myeloperoxidase* -Oxidized-low density lipoprotein |
MACROENVIRONMENT -Adipocytokines (adiponectin)* -Neutrophil gelatinase-associated lipocalin (NGAL)* -Beta-trace protein* -Cystatin-C* -Kidney injury molecule-1 -Endothelin -Adrenomedullin -MR-proADM* -Copeptin* -Growth differentiation factor-15* -Follistatin-like 1 |
Biomarker shown to have predictive abilities in cardiovascular outcomes
THE CARDIOMYOCYTE
Myocyte stress/stretch
One of the most well-described and studied biomarkers in heart failure and ventricular stress is B-type natriuretic peptide (BNP) and its amino terminal fragment (NT-pro-BNP). Activation of the BNP gene (natriuretic peptide B), in response to myocardial stress (predominantly via stretch) results in the production of both peptides. Both biomarkers diagnose acute heart failure syndromes [5, 6] but without predicting DCM or LV systolic dysfunction (LVSD). While NT-proBNP and BNP are both higher in patients with LVSD, in sub-studies modest correlations were seen predicting LVEF [7-9]. In a recent meta-analysis pooling biomarker-guided therapy trial data, a significant reduction in mortality was seen utilizing these natriuretic peptides in chronic heart failure management [10] but with varied baseline LVEF and with no specific analysis in DCM patients. Recently, however, the Use of NT-proBNP Testing to Guide Heart Failure Therapy in the Outpatient Setting study specifically looked at chronic LVSD (mean LVEF < 30%, of which a third had a non-ischemic etiology) and found a statistically significant reduction in total cardiovascular event rates, improved quality of life, and reduction in LV chamber size as shown by LV end-systolic and -diastolic volume indexes, thus indicating improved cardiac remodeling with NT-proBNP guided therapy compared to standard of care [11]. Moreover, recent advances in BNP physiology have targeted the up-stream 108 amino acid prohormone of BNP and NT-pro-BNP, pro-BNP1-108 to identify asymptomatic LVSD in conjunction to known predictive abilities of BNP and NT-proBNP [12-14].
Atrial natriuretic peptide (ANP), is secreted predominantly from the atria during times of stretch, although can be released from ventricular myocytes in times of ventricular stress [15]. ANP has recently been evaluated in the context of the precursor pro-ANP peptide using a sandwich assay of the mid regional sequence of ANP (MR-proANP), which is more stable and less susceptible to enzymatic degradation compared to ANP [16]. Two recent studies in patients with lower LVEF showed that elevated MR-proANP was able to identify LVSD and to predict mortality [17, 18].
Soluble ST-2 (sST2) is a truncated soluble receptor of the interleukin-1 receptor family and is a biomarker of mechanical strain and fibrosis in conjunction with IL-33 (a ligand of ST2, synthesized by cardiac fibroblasts) [19, 20]. In a small cohort of DCM patients, sST2 (in addition to NT-proBNP) predicted sudden cardiac death [21]. In acutely decompensated heart failure patients with depressed LVEF (median 34%), sST2 levels predicted mortality risk [22]. In an echo sub-study for the pro-BNP investigation of Dyspnea in the Emergency Room, sST2 levels correlated with cardiac structure (LV end-diastolic and systolic dimensions) in addition to predicting mortality [23], indicating the poor prognosis of cardiac remodeling and potentially the utility of sST2.
Myocyte injury
We will not discuss cardiac troponin I and T in this review, but refer the reader to Table 2 where other biomarkers not discussed in this review are listed. Cardiac troponins are the most common markers of myocyte necrosis. Release of troponin in heart failure is due to myocyte injury, regardless of the mechanism involved and low circulating levels are present in chronic LVSD and DCM [24, 25].
Table 2.
Additional Biomarkers in Dilated Cardiomyopathy (DCM)
| Inflammatory markers |
| • TNF-α116-118 |
| • C-reactive protein119,120 |
| • Interleukin (IL) 6117,120 , IL 10117,121,122, IL 18123-125 |
| • TNF-related apoptosis inducing ligand (TRAIL)30,126 |
| Neurohormones |
| • Norepinephrine127,128 |
| • Renin127-129 |
| • Aldosterone130,131 |
| • Angiotensin II132 |
| • Arginine vasopressin127,133,134 |
Myocyte apoptosis
Heart-type fatty acid binding protein (H-FABP) is a cytosolic, non-enzymatic protein, which transports long-chain fatty acids into cardiomyocytes and is released into the circulation when the myocardium is injured [26]. In heart failure patients, H-FABP levels together with cTnT, were more sensitive in detecting worse New York Heart Association (NYHA) functional class and identified a higher risk group when cTnT levels were normal but H-FABP levels were elevated [27]. Several studies showed that H-FABP levels were strongly predictive of cardiac mortality and heart failure re-hospitalizations in multivariate analyses [27-29]. Thus, its strength in prognosis is likely because this biomarker represents ongoing myocardial damage and identifies a higher risk subset and likely provides insights into ongoing adverse cardiac remodeling.
Similarly, soluble markers of apoptosis, specifically soluble apoptosis stimulating fragment (sFAS) may eventually allow earlier prognostication in patients with heart failure. In low LVEF patients, sFAS independently predicted the composite outcome of all-cause mortality and rehospitalization for worsening heart failure [30]. Inflammatory mediators, such as such as IL-6 and TNF-α (Table 2), appear to be important determinants of the FAS-FAS ligand pathway [31] – thus, the inflammatory milieu of heart failure contributes to the apoptotic process in patients with LVSD.
Myostatin (also known as growth differentiating factor-8, GDF-8), a member of the transforming growth factor-beta (TGF-β) superfamily, is a secreted factor that inhibits muscle differentiation and growth. Myostatin is produced predominantly in skeletal muscle and is a negative regulator of skeletal muscle mass [32]. However recent evidence now links myostatin to cardiomyocyte homeostasis, since myostatin is up-regulated in patients with severe heart failure and DCM. Increased myostatin levels are seen as a possible protective counter-regulator of cardiomyocyte hypertrophy in advanced heart failure [33, 34]. A recent study in patients with depressed LVEF and heart failure (mean LVEF 22.6%) showed increased myostatin levels correlated with worsening NYHA classification [35]. Whether myostatin serum levels correlates with the skeletal muscle wasting phenomenon in heart failure and cardiac cachexia, still remains to be studied.
THE MICROENVIRONMENT
Cardiac interstitium
In DCM, the myocardial interstitium is in a constant state of flux with increased extracellular matrix turnover and decreased collagen linking, leading to a distorted and defective matrix architecture [36, 37]. A loss of alignment of the cardiomyocyte fascicles contributes to LV dilation and a loss of the matrix-myocyte interface likely weakens the myocyte-shortening force transduction, leading to impaired myocardial force and systolic performance [38]. Matrix metalloproteinases (MMPs), myocardial interstitium proteases that are pivotal in myocardial remodeling, are held in delicate balance by tissue-inhibitors of matrix metalloproteinases (TIMPs) [39]. The overall change in matrix proteins in DCM favors increased MMP-mediated matrix proteolysis with up-regulation noted in MMP-2, MMP-3, MMP-9, MMP-13 and MT1-MMP with decreases seen in MMP-1 and varied responses of TIMP-1, TIMP-2, and TIMP-3. However, these findings were measured by tissue samples and not by blood protein characterization [36, 37, 40]. Plasma MMP-9 levels in the Randomized Evaluation of Strategies for LV Dysfunction (RESOLVED) study were inversely related to LV systolic function – suggesting a role for monitoring ongoing cardiac remodeling [41]. Plasma TIMP-1 levels correlated with cardiovascular risk factors, LV mass and hypertrophy and were inversely associated with cardiac function (fractional shortening) in a community-based cohort [42] – suggesting these proteins may provide early predictive information in patients at greatest risk for developing heart failure.
In addition to the regulators of collagen matrix formation, serum procollagen peptides have been evaluated to characterize and understand extracellular matrix turnover. Circulating amino-terminal propeptide of type III procollagen (PIIINP) portend a higher risk of death and hospitalization, particularly in lower LVEF patients [41]. Reverse remodeling after LV assist device implantation supports an initial increase in serum N-terminal pro-peptide for Type I collagen (PINP) and PIIINP which may provide insight to adverse cardiac remodeling [43]. Additional studies in a specific DCM cohort with serum markers are needed to further elucidate their role in risk prediction and prognosis.
Galectin-3, a β-galactoside-binding protein secreted by immune cells, has been associated with myocardial fibrosis, ventricular remodeling, and left ventricular dysfunction [44, 45]. In two recent studies evaluating chronic heart failure patients, galectin-3 predicted mortality and heart failure hospitalization and correlated positively with LV end-systolic and end-diastolic volumes [46, 47]. Similarly, galectin-3 was associated with all-cause mortality and an increased risk of incident heart failure, thus implicating the predictive role of galectin-3 in detecting asymptomatic fibrosis and early adverse remodeling [48].
Inflammation
Many markers of inflammation have been implicated in the pathogenesis of heart failure with TNF-α being one of the most well studied [49, 50]. We will not be discussing TNF-α in this review (see Table 2 for references).
Tumor necrosis factor-like weak inducer of apoptosis (TWEAK), a member of the TNF factor family, is a trans-membrane protein that is released in a truncated, active form to bind to fibroblast growth factor inducible-14 (Fn14). Fn14 is a highly inducible cell-surface receptor that is involved in multiple signaling pathways, including the NF-κB pathway. TWEAK plays a role in cardiomyocyte proliferation, myocardial hypertrophy, and cardiac fibrosis during cardiac remodeling. However there is conflicting data about the use of TWEAK levels as a biomarker. On the one hand increased levels of TWEAK after myocardial infarction with cardiac remodeling has been shown to portend adverse outcomes [51]. Conversely lower levels of soluble TWEAK have been associated with increased mortality in heart failure patients [52, 53]. Thus the role of TWEAK in heart failure and LVSD require further clarification.
Osteoprotegerin (OPG), a member of the TNF receptor superfamily, binds to the receptor activator of NK-κB ligand (RANKL) and prevents the interaction between RANKL and its receptor. In a cohort of stable heart failure patients with LVSD (mean LVEF 32%) with both ischemic and non-ischemic etiologies, OPG levels were elevated in heart failure patients compared to controls and were positively correlated with worse NYHA functional class, degree of myocardial dysfunction (cardiac index), and neurohormonal activation (N-BNP) [54]. Additionally, in chronic heart failure patients, OPG levels are associated with mortality, independent of other risk factors of death. Thus OPG levels may assist in risk stratification in these patients [55].
Pentraxin-3 (PTX3), a member of the pentraxin superfamily (which includes C-reactive protein and serum-amyloid P), is an inflammatory marker that is part of the innate immunity system which is produced by endothelial cells, smooth muscle cells, and macrophages [56]. PTX3, not C-reactive protein, proved to be an independent predictor of adverse events – including all-cause mortality and hospitalization for worsening heart failure in several heart failure studies including GISSI-heart failure and CORONA cohorts [57, 58].
Cardiotrophin-1 (CT-1), is a cytokine and a member of the IL-6 family of cytokines. CT-1 mediates its effects by interacting with the glycoprotein 130 (gp130)/leukemia inhibitory factor receptor beta (LIFR) heterodimer. It has potent hypertrophic and survival effects on cardiac myocytes. CT-1 activates phosphatidylinositol 3-kinase in cardiac myocytes and enhances transcription factor NF-κB DNA-binding activities. CT-1 levels are increased in patients with DCM and are significantly correlated with the LV mass index, suggesting that CT-1 plays an important role in structural LV remodeling [59].
Oxidative Stress
Increased oxidative stress is characterized by the excessive production of reactive oxygen species (ROS) which overwhelms the host’s antioxidant defenses. Oxidative stress is present in many cardiovascular disorders, such as heart failure. Increased ROS in heart failure may mediate many pathways that play a role in adverse cardiac remodeling including the propagation of apoptosis, deleterious effects on endothelial function, activation of neurohormonal systems [60], as well as direct effects on cardiomyocytes that can impair cardiac performance (i.e. ROS-induced structural modifications of the sarcomere) [61]. Many methods exist to characterized levels of ROS, however, only recently have there been human studies evaluating biomarkers that measure indirect markers of free radicals and assess their role in heart failure.
One biomarker of interest is myeloperoxidase (MPO), a leukocyte-derived heme peroxidase that is associated with neutrophil activation and inflammation with direct effects on ventricular remodeling in the post-infarct setting [62, 63]. In patients with LVEF < 35%, MPO was associated with right ventricular dysfunction and a more restrictive diastolic dysfunction pattern, on echocardiography. MPO was also more predictive of increased future adverse clinical events even after multivariate adjustment [64]. In addition, combined with other markers of inflammation (like high-sensitivity C-reactive protein) MPO levels provided incremental risk prediction in chronic systolic heart failure patients [64]. Thus MPO levels may also provide a means of monitoring anti-inflammatory effects with heart failure therapy [65] and determining on-going risk in heart failure patients.
Another marker of oxidative stress, plasma oxidized low-density lipoprotein (oxLDL) levels, was shown to be elevated in the coronary sinus of the heart compare to aortic root samples in DCM patients demonstrating increased oxidative stress in heart failure patients [66]. This transcardiac gradient of oxLDL correlated inversely with LV systolic function with oxLDL levels have been shown to be an independent predictor of mortality and adverse cardiac events in heart failure patients [66-68].
THE MACROENVIRONMENT
Myocyte energetics
Alterations in cardiac metabolism and energy substrate utilization have been described as pathological consequences as well as therapeutic targets for heart failure [69, 70]. Adipokines are secreted by adipose tissue and are implicated as playing a role in the pathogenesis of heart failure [71, 72]. Adiponectin, an insulin-sensitizing hormone, is secreted not only by adipose tissue but also cardiomyocytes during cardiac stress. Adiponectin activates AMP activated protein kinase (AMPK) leading to downstream effects such as inhibiting LV hypertrophy as well optimizing energetics by preferentially supporting the major cardiomyocyte fuel, fatty acids, which is responsible for ~70% of the cardiac energy needs [73]. Recent studies have implicated changes in serum adipokine levels between compensated and decompensated heart failure as well as evidence of adiponectin resistance that is reversed in the setting of left ventricular assist device (LVAD) therapy [74, 75]. Adiponectin modulates cardiac dysfunction by its interaction with several intracellular signaling pathways [76]. Depressed levels of adiponectin reflect greater cardiovascular risk and inflammation, in conditions such as hypertension, coronary artery disease, obesity and insulin resistance [77-79]. On the contrary, in humans with LVSD and heart failure [33, 80, 81], adiponectin levels are elevated [71] and associated with the severity of heart failure symptoms, disease severity and mortality [80, 82, 83]. It has been proposed that elevated adiponectin in LVSD is a state of ‘adiponectin-resistance’ and reflects an attempt to mitigate pro-inflammatory or impaired metabolic states and demonstrates a balance between protective and harmful pathways in the failing heart.
Neurohormones
Neurohormones such as norepinephrine, renin, angiotensin II and aldosterone (see Table 2 for references) have been well described in prior reviews of biomarkers in heart failure [60]. Novel neurohormones including adrenomedullin (ADM), a peptide which is released by many tissue types, including the kidney and adrenal medulla and is stimulated by pressure and volume overload. ADM belongs to the calcitonin gene-related peptide family and is implicated in vasodilation via nitric oxide with resultant cardioprotective effects such as increasing cardiac output, decreasing afterload, and modulating cardiac fibroblast proliferation [84]. Mid-region-proADM is a stable prohormone fragment of ADM that is easier to measure. It independently predicted 2-year mortality, particularly in LVSD with non-ischemic etiology and NYHA Class II or worse functional class [85].
Copeptin, the C-terminal portion of the precursor peptide of the neurohormone arginine vasopressin (AVP or antidiuretic hormone), is a surrogate biomarker for AVP, which exerts its primary effects in the hypothalamus by stimulating adrenocorticotrophic hormone, on vascular tissue causing vasoconstriction, and in the kidneys resulting in water retention. Increased copeptin levels in LVSD populations is linked to decreased survival rates, particularly in symptomatic NYHA class II-IV heart failure patients [86]. In the OPTIMAAL (Optimal Trial In Myocardial Infarction with Angiotensin II Antagonist Losartan) neurohormonal substudy which included a mixed population of acute heart failure syndrome plus LVSD patients, copeptin levels were a predictor of mortality post-myocardial infarction and performed better than BNP and NT-proBNP levels [87].
Renal markers
The relationship between renal dysfunction and heart failure is tightly intertwined with activation of neurohormones and release of stress biomarkers that are associated with adverse cardiac outcomes [88]. Neutrophil gelatinase-associated lipocalin (NGAL) is secreted by many cell types including renal tubule cells, hepatocytes, endothelial, and smooth muscle cells in response to cellular stress, inflammation and ischemia [89]. NGAL, in patients with acutely decompensated heart failure at the time of discharge, was a strong predictor of 30-day heart failure readmissions and all-cause mortality [90]. In addition, NGAL predicted acute kidney injury in heart failure patient with LVSD [91] and correlated strongly with markers of anemia even after multivariate adjustment, such as renal function and markers of inflammation [92], suggesting that NGAL initially produced as a compensatory response may not simply be a risk marker but an active player in the heart failure syndrome.
Beta-trace protein (BTP), a glycoprotein produced in all tissues (except the ovaries) converts prostaglandin H2 to prostaglandin D2 and cystatin C, a competitive inhibitor of cysteine proteases is produced by all nucleated cells. Both have been purported to be novel markers of glomerular filtration [93]. In acutely decompensated heart failure patients, both BTP and cystatin C predicted risk of death/and or heart failure hospitalizations and were superior to creatinine, estimated glomerular filtration rate, and blood urea nitrogen [93]. However, characterization of these biomarkers specifically in DCM has not been evaluated and merits further investigation.
Kidney injury molecule-1 (KIM-1) is also a glycoprotein that is expressed in the proximal tubule in renal injury. Both KIM-1 and NGAL levels were increased in patients with LVSD and may provide prognostic information in LVSD patients with mild renal insufficiency. These findings suggest an important role for biomarkers in cardiorenal interactions in heart failure [94].
Increasing evidence shows that the heart secretes factors to maintain its performance and coordinate cellular activities in response to cardiac stress as outlined above. However, newer proteins secreted from cardiac tissue have been identified. These cardiac-secreted factors are termed cardiokines.
Growth differentiation factor-15 (GDF-15) is a stress-responsive cytokine induced by cardiac stress. GDF-15 in conjunction with other biomarkers may add prognostic value for predicting death, overall cardiovascular events, and heart failure in community based studies [95]. Importantly elevated circulating levels of GDF-15 are evident in end-stage DCM patients at the time of LVAD implantation and levels decrease after mechanical unloading. GDF-15 correlates with myocardial fibrosis and kidney function [96].
Follistatin-like 1 (Fstl1), also referred to as TSC36 (TGFβ stimulating clone 36), is a divergent member of the Follistatin family of extracellular glycoproteins, and it functions in a non-canonical manner relative to other family members. Fstl1 is poorly understood with regard to its function, but has been shown to suppress cell growth and invasion. Fstl1 has both anti-inflammatory [97, 98] and pro-inflammatory [99] actions. Fstl1 is also regulated in human heart failure. In a collaborative effort between the Sam and Eschenhagen labs, we examined protein expression of Fstl1 in failing explanted human myocardium. Circulating Fstl1 levels were measured in a well-characterized cohort of chronic heart failure patients with LVSD. Fstl1 levels were significantly elevated and significantly associated with LVH and circulating levels of BNP [100]. Other groups have shown that myocardial Fstl1 mRNA levels are elevated in severe systolic heart failure that return to normal when LV function recovers after LVAD explantation [101].
In this review, newer and perhaps mechanistically more important biomarkers in DCM (and likely other cardiomyopathies) were selected, that have shown to confer predictive and prognostic abilities in symptomatic and asymptomatic heart failure patients. Importantly several of these biomarkers have been evaluated in other cardiomyopathies that have mechanistic overlap (e.g., increased fibrosis in heart failure with preserved ejection fraction (HFpEF), amyloidosis, hypertrophic cardiomyopathy etc). Of the aforementioned biomarkers, NT-proBNP, BNP, PTX-3, GDF-15, galectin-3 and ST-2 have been implicated in both the prediction of incident heart failure and also cardiovascular outcomes in HFpEF phenotypes [102-109]. Osteoprotegerin levels predict cardiovascular outcomes and incident heart failure events in patients with ischemia as well as in chronic ischemic heart failure [110, 111]. Mid-region pro-ADM levels have been shown to predict mortality in light chain cardiac amyloidosis [112]. KIM-1 levels have been implicated not only in LVSD but also in the prediction of incident HF [113]. Biomarkers, such as sFas, MPO, and follistatin-like 1 have been measured in patients with LVSD (and non-ischemic etiologies), but not specifically in DCM.
Thus, biomarkers in heart failure provide insight into the pathobiology of the cardiomyopathy and into mechanisms at the myocyte level, the micro- and macro-environment. Importantly, they provide diagnostic, predictive and prognostic information while offering opportunities for potential targets for emerging therapies for heart failure. Measurements of biomarkers, even those that are not independent risk predictors or specific to DCM, remain clinically important as they provide mechanistic information about the pathogenesis of heart failure. Although biomarkers have been studied and validated individually, recent studies have utilize multiple biomarker panels to help augment risk prediction, integrate multiple biologic pathways, and potentially increase specificity in heart failure groups. Ky and colleagues found that a score derived from multiple biomarkers (which encompassed diverse biologic pathways) improved the prediction of adverse events beyond current measures in heart failure patients with LVSD [114]. Similarly recent work from our group in patients with cardiac amyloidosis with LVSD, we showed that biomarkers in aggregate (MMP-2/TIMP-2 in combination with BNP and cTnI) had a potential discriminative ability for distinguishing between the different types of cardiac amyloidosis prompting more invasive diagnostic interventions and presumably therapies in these patients [115]. Furthermore assessment of these aggregate biomarkers suggests that therapeutic intervention that reduces collagen deposition should be studied in these patients [115]. Thus a greater focus and emphasis on multiple biomarker panels may be more important and clinically relevant, in terms of detection, prediction, diagnosis specificity, prognosis and therapeutic response. Combined with clinical assessments, biomarkers may lead to a better understanding of the various types of heart failure, allowing use of a more personalized approach in identifying and treating patients with LVSD and cardiomyopathy.
References
- 1.Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart A. Councilon Clinical Cardiology HF. Transplantation C. Quality of C. Outcomes R. Functional G. Translational Biology Interdisciplinary Working G. Council on E. Prevention Contemporary definitions and classification of the cardiomyopathies: An american heart association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113:1807–1816. doi: 10.1161/CIRCULATIONAHA.106.174287. [DOI] [PubMed] [Google Scholar]
- 2.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O’Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 world health organization/international society and federation of cardiology task force on the definition and classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ, Chamber Quantification Writing G. American Society of Echocardiography’s G. Standards C. European Association of E Recommendations for chamber quantification: A report from the american society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the european association of echocardiography, a branch of the european society of cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 4.Davies MJ, McKenna WJ. Dilated cardiomyopathy: An introduction to pathology and pathogenesis. Br Heart J. 1994;72:S24. doi: 10.1136/hrt.72.6_suppl.s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA. Breathing Not Properly Multinational Study I. Rapid measurement of b-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 6.Januzzi JL, Jr., Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, Lloyd-Jones DM, Brown DF, Foran-Melanson S, Sluss PM, Lee-Lewandrowski E, Lewandrowski KB. The n-terminal pro-bnp investigation of dyspnea in the emergency department (pride) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 7.Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT, Clopton P, Steg G, Aumont MC, Westheim A, Knudsen CW, Perez A, Kamin R, Kazanegra R, Herrmann HC, McCullough PA. Breathing Not Properly Multinational Study I Bedside b-type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the breathing not properly multinational study. J Am Coll Cardiol. 2003;41:2010–2017. doi: 10.1016/s0735-1097(03)00405-4. [DOI] [PubMed] [Google Scholar]
- 8.O’Donoghue M, Chen A, Baggish AL, Anwaruddin S, Krauser DG, Tung R, Januzzi JL. The effects of ejection fraction on n-terminal probnp and bnp levels in patients with acute chf: Analysis from the probnp investigation of dyspnea in the emergency department (pride) study. J Card Fail. 2005;11:S9–14. doi: 10.1016/j.cardfail.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 9.Steg PG, Joubin L, McCord J, Abraham WT, Hollander JE, Omland T, Mentre F, McCullough PA, Maisel AS. B-type natriuretic peptide and echocardiographic determination of ejection fraction in the diagnosis of congestive heart failure in patients with acute dyspnea. Chest. 2005;128:21–29. doi: 10.1378/chest.128.1.21. [DOI] [PubMed] [Google Scholar]
- 10.Felker GM, Hasselblad V, Hernandez AF, O’Connor CM. Biomarker-guided therapy in chronic heart failure: A meta-analysis of randomized controlled trials. Am Heart J. 2009;158:422–430. doi: 10.1016/j.ahj.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 11.Januzzi JL, Jr., Rehman SU, Mohammed AA, Bhardwaj A, Barajas L, Barajas J, Kim HN, Baggish AL, Weiner RB, Chen-Tournoux A, Marshall JE, Moore SA, Carlson WD, Lewis GD, Shin J, Sullivan D, Parks K, Wang TJ, Gregory SA, Uthamalingam S, Semigran MJ. Use of amino-terminal pro-b-type natriuretic peptide to guide outpatient therapy of patients with chronic left ventricular systolic dysfunction. J Am Coll Cardiol. 2011;58:1881–1889. doi: 10.1016/j.jacc.2011.03.072. [DOI] [PubMed] [Google Scholar]
- 12.Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, Sandberg S, Ikeda Y, Cataliotti A, Bailey K, Rodeheffer R, Burnett JC., Jr Pro-b-type natriuretic peptide(1-108) circulates in the general community: Plasma determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2011;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Costello-Boerrigter LC, Boerrigter G, Redfield MM, Rodeheffer RJ, Urban LH, Mahoney DW, Jacobsen SJ, Heublein DM, Burnett JC., Jr Amino-terminal pro-b-type natriuretic peptide and b-type natriuretic peptide in the general community: Determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47:345–353. doi: 10.1016/j.jacc.2005.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: Impact of age and gender. J Am Coll Cardiol. 2002;40:976–982. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura M, Yasue H, Ogawa H. Pathophysiological significance and clinical application of anp and bnp in patients with heart failure. Can J Physiol Pharmacol. 2001;79:730–735. [PubMed] [Google Scholar]
- 16.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204. [DOI] [PubMed] [Google Scholar]
- 17.Moertl D, Berger R, Struck J, Gleiss A, Hammer A, Morgenthaler NG, Bergmann A, Huelsmann M, Pacher R. Comparison of midregional pro-atrial and b-type natriuretic peptides in chronic heart failure: Influencing factors, detection of left ventricular systolic dysfunction, and prediction of death. J Am Coll Cardiol. 2009;53:1783–1790. doi: 10.1016/j.jacc.2009.01.057. [DOI] [PubMed] [Google Scholar]
- 18.von Haehling S, Jankowska EA, Morgenthaler NG, Vassanelli C, Zanolla L, Rozentryt P, Filippatos GS, Doehner W, Koehler F, Papassotiriou J, Kremastinos DT, Banasiak W, Struck J, Ponikowski P, Bergmann A, Anker SD. Comparison of midregional pro-atrial natriuretic peptide with n-terminal pro-b-type natriuretic peptide in predicting survival in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1973–1980. doi: 10.1016/j.jacc.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Weinberg EO, Shimpo M, DeKeulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of st2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. Il-33 and st2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pascual-Figal DA, Ordonez-Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes-Genis A, Investigators M Soluble st2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. doi: 10.1016/j.jacc.2009.07.041. [DOI] [PubMed] [Google Scholar]
- 22.Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High-sensitivity st2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. doi: 10.1161/CIRCHEARTFAILURE.110.958223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah RV, Chen-Tournoux AA, Picard MH, van Kimmenade RR, Januzzi JL. Serum levels of the interleukin-1 receptor family member st2, cardiac structure and function, and long-term mortality in patients with acute dyspnea. Circ Heart Fail. 2009;2:311–319. doi: 10.1161/CIRCHEARTFAILURE.108.833707. [DOI] [PubMed] [Google Scholar]
- 24.Jungbauer CG, Riedlinger J, Buchner S, Birner C, Resch M, Lubnow M, Debl K, Buesing M, Huedig H, Riegger G, Luchner A. High-sensitive troponin t in chronic heart failure correlates with severity of symptoms, left ventricular dysfunction and prognosis independently from n-terminal pro-b-type natriuretic peptide. Clin Chem Lab Med. 2011;49:1899–1906. doi: 10.1515/CCLM.2011.251. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Fujiwara H, Takatsu Y. Cardiac troponin and heart failure in the era of high-sensitivity assays. J Cardiol. 2012;60:160–167. doi: 10.1016/j.jjcc.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Schaap FG, van der Vusse GJ, Glatz JF. Fatty acid-binding proteins in the heart. Mol Cell Biochem. 1998;180:43–51. [PubMed] [Google Scholar]
- 27.Niizeki T, Takeishi Y, Arimoto T, Takabatake N, Nozaki N, Hirono O, Watanabe T, Nitobe J, Harada M, Suzuki S, Koyama Y, Kitahara T, Sasaki T, Kubota I. Heart-type fatty acid-binding protein is more sensitive than troponin t to detect the ongoing myocardial damage in chronic heart failure patients. J Card Fail. 2007;13:120–127. doi: 10.1016/j.cardfail.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 28.Setsuta K, Seino Y, Ogawa T, Arao M, Miyatake Y, Takano T. Use of cytosolic and myofibril markers in the detection of ongoing myocardial damage in patients with chronic heart failure. Am J Med. 2002;113:717–722. doi: 10.1016/s0002-9343(02)01394-3. [DOI] [PubMed] [Google Scholar]
- 29.Arimoto T, Takeishi Y, Shiga R, Fukui A, Tachibana H, Nozaki N, Hirono O, Nitobe J, Miyamoto T, Hoit BD, Kubota I. Prognostic value of elevated circulating heart-type fatty acid binding protein in patients with congestive heart failure. J Card Fail. 2005;11:56–60. doi: 10.1016/j.cardfail.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Niessner A, Hohensinner PJ, Rychli K, Neuhold S, Zorn G, Richter B, Hulsmann M, Berger R, Mortl D, Huber K, Wojta J, Pacher R. Prognostic value of apoptosis markers in advanced heart failure patients. Eur Heart J. 2009;30:789–796. doi: 10.1093/eurheartj/ehp004. [DOI] [PubMed] [Google Scholar]
- 31.Kinugawa T, Kato M, Yamamoto K, Hisatome I, Nohara R. Proinflammatory cytokine activation is linked to apoptotic mediator, soluble fas level in patients with chronic heart failure. Int Heart J. 2012;53:182–186. doi: 10.1536/ihj.53.182. [DOI] [PubMed] [Google Scholar]
- 32.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new tgf-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 33.George I, Bish LT, Kamalakkannan G, Petrilli CM, Oz MC, Naka Y, Sweeney HL, Maybaum S. Myostatin activation in patients with advanced heart failure and after mechanical unloading. Eur J Heart Fail. 2010;12:444–453. doi: 10.1093/eurjhf/hfq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma M, Kambadur R, Matthews KG, Somers WG, Devlin GP, Conaglen JV, Fowke PJ, Bass JJ. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol. 1999;180:1–9. doi: 10.1002/(SICI)1097-4652(199907)180:1<1::AID-JCP1>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 35.Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Increased plasma myostatin in heart failure. Eur J Heart Fail. 2011;13:734–736. doi: 10.1093/eurjhf/hfr024. [DOI] [PubMed] [Google Scholar]
- 36.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in lv myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 37.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 38.Eckhouse SR, Spinale FG. Changes in the myocardial interstitium and contribution to the progression of heart failure. Heart Fail Clin. 2012;8:7–20. doi: 10.1016/j.hfc.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 40.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 41.Cicoira M, Rossi A, Bonapace S, Zanolla L, Golia G, Franceschini L, Caruso B, Marino PN, Zardini P. Independent and additional prognostic value of aminoterminal propeptide of type iii procollagen circulating levels in patients with chronic heart failure. J Card Fail. 2004;10:403–411. doi: 10.1016/j.cardfail.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total timp-1 levels to cardiovascular risk factors and echocardiographic measures: The framingham heart study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 43.Bruggink AH, van Oosterhout MF, de Jonge N, Ivangh B, van Kuik J, Voorbij RH, Cleutjens JP, Gmelig-Meyling FH, de Weger RA. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. J Heart Lung Transplant. 2006;25:1091–1098. doi: 10.1016/j.healun.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Liu YH, D’Ambrosio M, Liao TD, Peng H, Rhaleb NE, Sharma U, Andre S, Gabius HJ, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404–412. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Andres N, Rossignol P, Iraqi W, Fay R, Nuee J, Ghio S, Cleland JG, Zannad F, Lacolley P. Association of galectin-3 and fibrosis markers with long-term cardiovascular outcomes in patients with heart failure, left ventricular dysfunction, and dyssynchrony: Insights from the care-hf (cardiac resynchronization in heart failure) trial. Eur J Heart Fail. 2012;14:74–81. doi: 10.1093/eurjhf/hfr151. [DOI] [PubMed] [Google Scholar]
- 47.Lok DJ, Lok SI, Bruggink-Andre de la Porte PW, Badings E, Lipsic E, van Wijngaarden J, de Boer RA, van Veldhuisen DJ, van de rMeer P. Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol. 2012 doi: 10.1007/s00392-012-0500-y. [DOI] [PubMed] [Google Scholar]
- 48.Ho JE, Liu C, Lyass A, Courchesne P, Pencina MJ, Vasan RS, Larson MG, Levy D. Galectin-3, a marker of cardiac fibrosis, predicts incident heart failure in the community. J Am Coll Cardiol. 2012;60:1249–1256. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. doi: 10.1056/NEJM199007263230405. [DOI] [PubMed] [Google Scholar]
- 50.Mann DL. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ Res. 2002;91:988–998. doi: 10.1161/01.res.0000043825.01705.1b. [DOI] [PubMed] [Google Scholar]
- 51.Ren MY, Sui SJ. The role of tweak/fn14 in cardiac remodeling. Mol Biol Rep. 2012;39:9971–9977. doi: 10.1007/s11033-012-1867-6. [DOI] [PubMed] [Google Scholar]
- 52.Chorianopoulos E, Rosenberg M, Zugck C, Wolf J, Katus HA, Frey N. Decreased soluble tweak levels predict an adverse prognosis in patients with chronic stable heart failure. Eur J Heart Fail. 2009;11:1050–1056. doi: 10.1093/eurjhf/hfp139. [DOI] [PubMed] [Google Scholar]
- 53.Richter B, Rychli K, Hohensinner PJ, Berger R, Mortl D, Neuhold S, Zorn G, Huber K, Maurer G, Wojta J, Pacher R, Hulsmann M, Niessner A. Differences in the predictive value of tumor necrosis factor-like weak inducer of apoptosis (tweak) in advanced ischemic and non-ischemic heart failure. Atherosclerosis. 2010;213:545–548. doi: 10.1016/j.atherosclerosis.2010.08.061. [DOI] [PubMed] [Google Scholar]
- 54.Ueland T, Yndestad A, Oie E, Florholmen G, Halvorsen B, Froland SS, Simonsen S, Christensen G, Gullestad L, Aukrust P. Dysregulated osteoprotegerin/rank ligand/rank axis in clinical and experimental heart failure. Circulation. 2005;111:2461–2468. doi: 10.1161/01.CIR.0000165119.62099.14. [DOI] [PubMed] [Google Scholar]
- 55.Roysland R, Masson S, Omland T, Milani V, Bjerre M, Flyvbjerg A, DiTano G, Misuraca G, Maggioni AP, Tognoni G, Tavazzi L, Latini R, Investigators G-H Prognostic value of osteoprotegerin in chronic heart failure: The gissi-hf trial. Am Heart J. 2010;160:286–293. doi: 10.1016/j.ahj.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 56.Bottazzi B, Doni A, Garlanda C, Mantovani A. An integrated view of humoral innate immunity: Pentraxins as a paradigm. Annu Rev Immunol. 2010;28:157–183. doi: 10.1146/annurev-immunol-030409-101305. [DOI] [PubMed] [Google Scholar]
- 57.Latini R, Gullestad L, Masson S, Nymo SH, Ueland T, Cuccovillo I, Vardal M, Bottazzi B, Mantovani A, Lucci D, Masuda N, Sudo Y, Wikstrand J, Tognoni G, Aukrust P, Tavazzi L, Investigators of the Controlled Rosuvastatin Multinational Trial in Heart F. trials GI-HF Pentraxin-3 in chronic heart failure: The corona and gissi-hf trials. Eur J Heart Fail. 2012;14:992–999. doi: 10.1093/eurjhf/hfs092. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T, Sagara M, Kubota I. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J. 2008;155:75–81. doi: 10.1016/j.ahj.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 59.Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Fujii M, Matsumoto T, Yamamoto T, Wang X, Asai S, Tsuji T, Tanaka H, Saito Y, Kuwahara K, Nakao K, Kinoshita M. Relationship between plasma level of cardiotrophin-1 and left ventricular mass index in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;38:1485–1490. doi: 10.1016/s0735-1097(01)01576-5. [DOI] [PubMed] [Google Scholar]
- 60.Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358:2148–2159. doi: 10.1056/NEJMra0800239. [DOI] [PubMed] [Google Scholar]
- 61.Steinberg SF. Oxidative stress and sarcomeric proteins. Circ Res. 2013;112:393–405. doi: 10.1161/CIRCRESAHA.111.300496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vasilyev N, Williams T, Brennan ML, Unzek S, Zhou X, Heinecke JW, Spitz DR, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase-generated oxidants modulate left ventricular remodeling but not infarct size after myocardial infarction. Circulation. 2005;112:2812–2820. doi: 10.1161/CIRCULATIONAHA.105.542340. [DOI] [PubMed] [Google Scholar]
- 63.Nicholls SJ, Hazen SL. Myeloperoxidase and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1102–1111. doi: 10.1161/01.ATV.0000163262.83456.6d. [DOI] [PubMed] [Google Scholar]
- 64.Tang WH, Tong W, Troughton RW, Martin MG, Shrestha K, Borowski A, Jasper S, Hazen SL, Klein AL. Prognostic value and echocardiographic determinants of plasma myeloperoxidase levels in chronic heart failure. J Am Coll Cardiol. 2007;49:2364–2370. doi: 10.1016/j.jacc.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 65.Andreou I, Tousoulis D, Miliou A, Tentolouris C, Zisimos K, Gounari P, Siasos G, Papageorgiou N, Papadimitriou CA, Dimopoulos MA, Stefanadis C. Effects of rosuvastatin on myeloperoxidase levels in patients with chronic heart failure: A randomized placebo-controlled study. Atherosclerosis. 2010;210:194–198. doi: 10.1016/j.atherosclerosis.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 66.Tsutamoto T, Wada A, Matsumoto T, Maeda K, Mabuchi N, Hayashi M, Tsutsui T, Ohnishi M, Sawaki M, Fujii M, Yamamoto T, Horie H, Sugimoto Y, Kinoshita M. Relationship between tumor necrosis factor-alpha production and oxidative stress in the failing hearts of patients with dilated cardiomyopathy. J Am Coll Cardiol. 2001;37:2086–2092. doi: 10.1016/s0735-1097(01)01299-2. [DOI] [PubMed] [Google Scholar]
- 67.Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: The impact of oxidative stress. Circ Heart Fail. 2009;2:608–615. doi: 10.1161/CIRCHEARTFAILURE.109.868513. [DOI] [PubMed] [Google Scholar]
- 68.Tsutsui T, Tsutamoto T, Wada A, Maeda K, Mabuchi N, Hayashi M, Ohnishi M, Kinoshita M. Plasma oxidized low-density lipoprotein as a prognostic predictor in patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;39:957–962. doi: 10.1016/s0735-1097(02)01721-7. [DOI] [PubMed] [Google Scholar]
- 69.Taegtmeyer H. Cardiac metabolism as a target for the treatment of heart failure. Circulation. 2004;110:894–896. doi: 10.1161/01.CIR.0000139340.88769.D5. [DOI] [PubMed] [Google Scholar]
- 70.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 71.Biolo A, Shibata R, Ouchi N, Kihara S, Sonoda M, Walsh K, Sam F. Determinants of adiponectin levels in patients with chronic systolic heart failure. Am J Cardiol. 2010;105:1147–1152. doi: 10.1016/j.amjcard.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Frankel DS, Vasan RS, D’Agostino RB, Sr., Benjamin EJ, Levy D, Wang TJ, Meigs JB. Resistin, adiponectin, and risk of heart failure the framingham offspring study. J Am Coll Cardiol. 2009;53:754–762. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nanayakkara G, Kariharan T, Wang L, Zhong J, Amin R. The cardio-protective signaling and mechanisms of adiponectin. Am J Cardiovasc Dis. 2012;2:253–266. [PMC free article] [PubMed] [Google Scholar]
- 74.Khan RS, Kato TS, Chokshi A, Chew M, Yu S, Wu C, Singh P, Cheema FH, Takayama H, Harris C, Reyes-Soffer G, Knoll R, Milting H, Naka Y, Mancini D, Schulze PC. Adipose tissue inflammation and adiponectin resistance in patients with advanced heart failure: Correction after ventricular assist device implantation. Circ Heart Fail. 2012;5:340–348. doi: 10.1161/CIRCHEARTFAILURE.111.964031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schulze PC, Biolo A, Gopal D, Shahzad K, Balog J, Fish M, Siwik D, Colucci WS. Dynamics in insulin resistance and plasma levels of adipokines in patients with acute decompensated and chronic stable heart failure. J Card Fail. 2011;17:1004–1011. doi: 10.1016/j.cardfail.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 78.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, Fu Y, Motone M, Yamamoto K, Matsuo A, Ohashi K, Kihara S, Funahashi T, Rakugi H, Matsuzawa Y, Ogihara T. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 79.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, Arita Y, Okamoto Y, Shimomura I, Hiraoka H, Nakamura T, Funahashi T, Matsuzawa Y, Osaka CADSGCad Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 80.Kistorp C, Faber J, Galatius S, Gustafsson F, Frystyk J, Flyvbjerg A, Hildebrandt P. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112:1756–1762. doi: 10.1161/CIRCULATIONAHA.104.530972. [DOI] [PubMed] [Google Scholar]
- 81.Haugen E, Furukawa Y, Isic A, Fu M. Increased adiponectin level in parallel with increased nt-pro bnp in patients with severe heart failure in the elderly: A hospital cohort study. Int J Cardiol. 2008;125:216–219. doi: 10.1016/j.ijcard.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 82.George J, Patal S, Wexler D, Sharabi Y, Peleg E, Kamari Y, Grossman E, Sheps D, Keren G, Roth A. Circulating adiponectin concentrations in patients with congestive heart failure. Heart. 2006;92:1420–1424. doi: 10.1136/hrt.2005.083345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takano H, Obata JE, Kodama Y, Kitta Y, Nakamura T, Mende A, Kawabata K, Saito Y, Fujioka D, Kobayashi T, Yano T, Sano K, Kugiyama K. Adiponectin is released from the heart in patients with heart failure. Int J Cardiol. 2009;132:221–226. doi: 10.1016/j.ijcard.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 84.Potocki M, Ziller R, Mueller C. Mid-regional pro-adrenomedullin in acute heart failure: A better biomarker or just another biomarker? Curr Heart Fail Rep. 2012;9:244–251. doi: 10.1007/s11897-012-0096-6. [DOI] [PubMed] [Google Scholar]
- 85.Adlbrecht C, Hulsmann M, Strunk G, Berger R, Mortl D, Struck J, Morgenthaler NG, Bergmann A, Jakowitsch J, Maurer G, Lang IM, Pacher R. Prognostic value of plasma midregional pro-adrenomedullin and c-terminal-pro-endothelin-1 in chronic heart failure outpatients. Eur J Heart Fail. 2009;11:361–366. doi: 10.1093/eurjhf/hfp004. [DOI] [PubMed] [Google Scholar]
- 86.Neuhold S, Huelsmann M, Strunk G, Stoiser B, Struck J, Morgenthaler NG, Bergmann A, Moertl D, Berger R, Pacher R. Comparison of copeptin, b-type natriuretic peptide, and amino-terminal pro-b-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J Am Coll Cardiol. 2008;52:266–272. doi: 10.1016/j.jacc.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 87.Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O, Bergmann A, Squire I, van Veldhuisen DJ, Dickstein K, Investigators O. C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: Results from the optimaal study. Eur Heart J. 2009;30:1187–1194. doi: 10.1093/eurheartj/ehp098. [DOI] [PubMed] [Google Scholar]
- 88.Carubelli V, Metra M, Lombardi C, Bettari L, Bugatti S, Lazzarini V, DeiCas L. Renal dysfunction in acute heart failure: Epidemiology, mechanisms and assessment. Heart Fail Rev. 2012;17:271–282. doi: 10.1007/s10741-011-9265-z. [DOI] [PubMed] [Google Scholar]
- 89.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, Barasch J. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18:407–413. doi: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 90.Maisel AS, Mueller C, Fitzgerald R, Brikhan R, Hiestand BC, Iqbal N, Clopton P, van Veldhuisen DJ. Prognostic utility of plasma neutrophil gelatinase-associated lipocalin in patients with acute heart failure: The ngal evaluation along with b-type natriuretic peptide in acutely decompensated heart failure (gallant) trial. Eur J Heart Fail. 2011;13:846–851. doi: 10.1093/eurjhf/hfr087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shrestha K, Shao Z, Singh D, Dupont M, Tang WH. Relation of systemic and urinary neutrophil gelatinase-associated lipocalin levels to different aspects of impaired renal function in patients with acute decompensated heart failure. Am J Cardiol. 2012;110:1329–1335. doi: 10.1016/j.amjcard.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shrestha K, Borowski AG, Troughton RW, Klein AL, Tang WH. Association between systemic neutrophil gelatinase-associated lipocalin and anemia, relative hypochromia, and inflammation in chronic systolic heart failure. Congest Heart Fail. 2012;18:239–244. doi: 10.1111/j.1751-7133.2012.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen HH. Beta-trace protein versus cystatin c: Which is a better surrogate marker of renal function versus prognostic indicator in cardiovascular diseases? J Am Coll Cardiol. 2011;57:859–860. doi: 10.1016/j.jacc.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Damman K, Van Veldhuisen DJ, Navis G, Vaidya VS, Smilde TD, Westenbrink BD, Bonventre JV, Voors AA, Hillege HL. Tubular damage in chronic systolic heart failure is associated with reduced survival independent of glomerular filtration rate. Heart. 2010;96:1297–1302. doi: 10.1136/hrt.2010.194878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: The framingham heart study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lok SI, Winkens B, Goldschmeding R, van Geffen AJ, Nous FM, van Kuik J, van der Weide P, Klopping C, Kirkels JH, Lahpor JR, Doevendans PA, de Jonge N, de Weger RA. Circulating growth differentiation factor-15 correlates with myocardial fibrosis in patients with non-ischaemic dilated cardiomyopathy and decreases rapidly after left ventricular assist device support. Eur J Heart Fail. 2012;14:1249–1256. doi: 10.1093/eurjhf/hfs120. [DOI] [PubMed] [Google Scholar]
- 97.Kawabata D, Tanaka M, Fujii T, Umehara H, Fujita Y, Yoshifuji H, Mimori T, Ozaki S. Ameliorative effects of follistatin-related protein/tsc-36/fstl1 on joint inflammation in a mouse model of arthritis. Arthritis Rheum. 2004;50:660–668. doi: 10.1002/art.20023. [DOI] [PubMed] [Google Scholar]
- 98.Le Luduec JB, Condamine T, Louvet C, Thebault P, Heslan JM, Heslan M, Chiffoleau E, Cuturi MC. An immunomodulatory role for follistatin-like 1 in heart allograft transplantation. Am J Transplant. 2008;8:2297–2306. doi: 10.1111/j.1600-6143.2008.02398.x. [DOI] [PubMed] [Google Scholar]
- 99.Miyamae T, Marinov AD, Sowders D, Wilson DC, Devlin J, Boudreau R, Robbins P, Hirsch R. Follistatin-like protein-1 is a novel proinflammatory molecule. J Immunol. 2006;177:4758–4762. doi: 10.4049/jimmunol.177.7.4758. [DOI] [PubMed] [Google Scholar]
- 100.El-Armouche A, Ouchi N, Tanaka K, Doros G, Wittkopper K, Schulze T, Eschenhagen T, Walsh K, Sam F. Follistatin-like 1 in chronic systolic heart failure: A marker of left ventricular remodeling. Circ Heart Fail. 2011;4:621–627. doi: 10.1161/CIRCHEARTFAILURE.110.960625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lara-Pezzi E, Felkin LE, Birks EJ, Sarathchandra P, Panse KD, George R, Hall JL, Yacoub MH, Rosenthal N, Barton PJ. Expression of follistatin-related genes is altered in heart failure. Endocrinology. 2008;149:5822–5827. doi: 10.1210/en.2008-0151. [DOI] [PubMed] [Google Scholar]
- 102.de Boer RA, Lok DJ, Jaarsma T, van der Meer P, Voors AA, Hillege HL, van Veldhuisen DJ. Predictive value of plasma galectin-3 levels in heart failure with reduced and preserved ejection fraction. Ann Med. 2011;43:60–68. doi: 10.3109/07853890.2010.538080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grewal J, McKelvie R, Lonn E, Tait P, Carlsson J, Gianni M, Jarnert C, Persson H. Bnp and nt-probnp predict echocardiographic severity of diastolic dysfunction. Eur J Heart Fail. 2008;10:252–259. doi: 10.1016/j.ejheart.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 104.Grewal J, McKelvie RS, Persson H, Tait P, Carlsson J, Swedberg K, Ostergren J, Lonn E. Usefulness of n-terminal pro-brain natriuretic peptide and brain natriuretic peptide to predict cardiovascular outcomes in patients with heart failure and preserved left ventricular ejection fraction. Am J Cardiol. 2008;102:733–737. doi: 10.1016/j.amjcard.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 105.Manzano-Fernandez S, Mueller T, Pascual-Figal D, Truong QA, Januzzi JL. Usefulness of soluble concentrations of interleukin family member st2 as predictor of mortality in patients with acutely decompensated heart failure relative to left ventricular ejection fraction. Am J Cardiol. 2011;107:259–267. doi: 10.1016/j.amjcard.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, Matsuzawa Y, Akiyama E, Yamamoto E, Sakamoto K, Nagayoshi Y, Kaikita K, Sumida H, Kim-Mitsuyama S, Ogawa H. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 107.McKelvie RS, Komajda M, McMurray J, Zile M, Ptaszynska A, Donovan M, Carson P, Massie BM, Investigators IP Baseline plasma nt-probnp and clinical characteristics: Results from the irbesartan in heart failure with preserved ejection fraction trial. J Card Fail. 2010;16:128–134. doi: 10.1016/j.cardfail.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 108.Chu JW, Jones GT, Tarr GP, Phillips LV, Wilkins GT, van Rij AM, Williams MJ. Plasma active matrix metalloproteinase 9 and indices of diastolic function in patients with preserved systolic function. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.03.147. [DOI] [PubMed] [Google Scholar]
- 109.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: Improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–197. doi: 10.1093/eurjhf/hfn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Roysland R, Bonaca MP, Omland T, Sabatine M, Murphy SA, Scirica BM, Bjerre M, Flyvbjerg A, Braunwald E, Morrow DA. Osteoprotegerin and cardiovascular mortality in patients with non-st elevation acute coronary syndromes. Heart. 2012;98:786–791. doi: 10.1136/heartjnl-2011-301260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ueland T, Dahl CP, Kjekshus J, Hulthe J, Bohm M, Mach F, Goudev A, Lindberg M, Wikstrand J, Aukrust P, Gullestad L. Osteoprotegerin predicts progression of chronic heart failure: Results from corona. Circ Heart Fail. 2011;4:145–152. doi: 10.1161/CIRCHEARTFAILURE.110.957332. [DOI] [PubMed] [Google Scholar]
- 112.Palladini G, Barassi A, Perlini S, Milani P, Foli A, Russo P, Albertini R, Obici L, Lavatelli F, Sarais G, Casarini S, Moratti R, Melzi d’Eril GV, Merlini G. Midregional proadrenomedullin (mr-proadm) is a powerful predictor of early death in al amyloidosis. Amyloid. 2011;18:216–221. doi: 10.3109/13506129.2011.627069. [DOI] [PubMed] [Google Scholar]
- 113.Carlsson AC, Larsson A, Helmersson-Karlqvist J, Lind L, Ingelsson E, Larsson TE, Sundstrom J, Arnlov J. Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. Eur J Heart Fail. 2012 doi: 10.1093/eurjhf/hfs187. [DOI] [PubMed] [Google Scholar]
- 114.Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AH, Goldberg LR, Jessup M, Cappola TP. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–190. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tanaka H EE, Doros G, Tanriverdi K, Connors LH, Seldin DC, Sam F. Circulating matrix metalloproteinases and tissue inhibitors of metalloproteinases in cardiac amyloidosis. Journal of the American Heart Association. 2013 doi: 10.1161/JAHA.112.005868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amir O, Rogowski O, David M, Lahat N, Wolff R, Lewis BS. Circulating interleukin-10: Association with higher mortality in systolic heart failure patients with elevated tumor necrosis factor-alpha. Isr Med Assoc J. 2010;12:158–162. [PubMed] [Google Scholar]
- 117.Miettinen KH, Lassus J, Harjola VP, Siirila-Waris K, Melin J, Punnonen KR, Nieminen MS, Laakso M, Peuhkurinen KJ. Prognostic role of pro- and anti-inflammatory cytokines and their polymorphisms in acute decompensated heart failure. Eur J Heart Fail. 2008;10:396–403. doi: 10.1016/j.ejheart.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 118.Savic-Radojevic A, Radovanovic S, Pekmezovic T, Pljesa-Ercegovac M, Simic D, Djukic T, Matic M, Simic T. The role of serum vcam-1 and tnf-alpha as predictors of mortality and morbidity in patients with chronic heart failure. J Clin Lab Anal. 2013 doi: 10.1002/jcla.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Gennaro L, Brunetti ND, Cuculo A, Pellegrino PL, Di Biase M. Systemic inflammation in nonischemic dilated cardiomyopathy. Heart Vessels. 2008;23:445–450. doi: 10.1007/s00380-008-1075-4. [DOI] [PubMed] [Google Scholar]
- 120.Matsumoto M, Tsujino T, Lee-Kawabata M, Naito Y, Sakoda T, Ohyanagi M, Masuyama T. Serum interleukin-6 and c-reactive protein are markedly elevated in acute decompensated heart failure patients with left ventricular systolic dysfunction. Cytokine. 2010;49:264–268. doi: 10.1016/j.cyto.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 121.Kosar F, Aksoy Y, Ozguntekin G, Ozerol I, Varol E. Relationship between cytokines and tumour markers in patients with chronic heart failure. Eur J Heart Fail. 2006;8:270–274. doi: 10.1016/j.ejheart.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Wykretowicz A, Furmaniuk J, Smielecki J, Deskur-Smielecka E, Szczepanik A, Banaszak A, Wysocki H. The oxygen stress index and levels of circulating interleukin-10 and interleukin-6 in patients with chronic heart failure. Int J Cardiol. 2004;94:283–287. doi: 10.1016/j.ijcard.2003.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Eslick GD, Thampan BV, Nalos M, McLean AS, Sluyter R. Circulating interleukin-18 concentrations and a loss-of-function p2×7 polymorphism in heart failure. Int J Cardiol. 2009;137:81–83. doi: 10.1016/j.ijcard.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 124.Naito Y, Tsujino T, Fujioka Y, Ohyanagi M, Okamura H, Iwasaki T. Increased circulating interleukin-18 in patients with congestive heart failure. Heart. 2002;88:296–297. doi: 10.1136/heart.88.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamaoka-Tojo M, Tojo T, Inomata T, Machida Y, Osada K, Izumi T. Circulating levels of interleukin 18 reflect etiologies of heart failure: Th1/th2 cytokine imbalance exaggerates the pathophysiology of advanced heart failure. J Card Fail. 2002;8:21–27. doi: 10.1054/jcaf.2002.31628. [DOI] [PubMed] [Google Scholar]
- 126.Osmancik P, Teringova E, Tousek P, Paulu P, Widimsky P. Prognostic value of tnf-related apoptosis inducing ligand (trail) in acute coronary syndrome patients. PLoS One. 2013;8:e53860. doi: 10.1371/journal.pone.0053860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Francis GS, Benedict C, Johnstone DE, Kirlin PC, Nicklas J, Liang CS, Kubo SH, Rudin-Toretsky E, Yusuf S. Comparison of neuroendocrine activation in patients with left ventricular dysfunction with and without congestive heart failure. A substudy of the studies of left ventricular dysfunction (solvd) Circulation. 1990;82:1724–1729. doi: 10.1161/01.cir.82.5.1724. [DOI] [PubMed] [Google Scholar]
- 128.Latini R, Masson S, Anand I, Salio M, Hester A, Judd D, Barlera S, Maggioni AP, Tognoni G, Cohn JN, Val-He FTI. The comparative prognostic value of plasma neurohormones at baseline in patients with heart failure enrolled in val-heft. Eur Heart J. 2004;25:292–299. doi: 10.1016/j.ehj.2003.10.030. [DOI] [PubMed] [Google Scholar]
- 129.Vergaro G, Emdin M, Iervasi A, Zyw L, Gabutti A, Poletti R, Mammini C, Giannoni A, Fontana M, Passino C. Prognostic value of plasma renin activity in heart failure. Am J Cardiol. 2011;108:246–251. doi: 10.1016/j.amjcard.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 130.Vantrimpont P, Rouleau JL, Ciampi A, Harel F, de Champlain J, Bichet D, Moye LA, Pfeffer M. Two-year time course and significance of neurohumoral activation in the survival and ventricular enlargement (save) study. Eur Heart J. 1998;19:1552–1563. doi: 10.1053/euhj.1998.1093. [DOI] [PubMed] [Google Scholar]
- 131.Cicoira M, Zanolla L, Franceschini L, Rossi A, Golia G, Zeni P, Caruso B, Zardini P. Relation of aldosterone “escape” despite angiotensin-converting enzyme inhibitor administration to impaired exercise capacity in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2002;89:403–407. doi: 10.1016/s0002-9149(01)02261-5. [DOI] [PubMed] [Google Scholar]
- 132.Roig E, Perez-Villa F, Morales M, Jimenez W, Orus J, Heras M, Sanz G. Clinical implications of increased plasma angiotensin ii despite ace inhibitor therapy in patients with congestive heart failure. Eur Heart J. 2000;21:53–57. doi: 10.1053/euhj.1999.1740. [DOI] [PubMed] [Google Scholar]
- 133.Goldsmith SR, Francis GS, Cowley AW, Jr., Levine TB, Cohn JN. Increased plasma arginine vasopressin levels in patients with congestive heart failure. J Am Coll Cardiol. 1983;1:1385–1390. doi: 10.1016/s0735-1097(83)80040-0. [DOI] [PubMed] [Google Scholar]
- 134.Szatalowicz VL, Arnold PE, Chaimovitz C, Bichet D, Berl T, Schrier RW. Radioimmunoassay of plasma arginine vasopressin in hyponatremic patients with congestive heart failure. N Engl J Med. 1981;305:263–266. doi: 10.1056/NEJM198107303050506. [DOI] [PubMed] [Google Scholar]