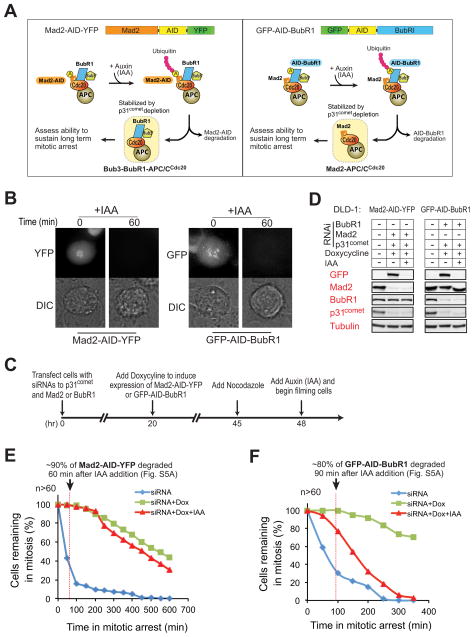
Figure 5. BubR1, not Mad2, is required to sustain inhibition of APC/CCdc20 in vivo.
(A) Schematic for the inducible destruction of Mad2-AID-YFP or GFP-AID-BubR1 in mitotic cells using an Auxin-inducible protein degradation system. (B) Microscopic images showing Auxin (indoleacetic acid, IAA)-induced mitotic degradation of Mad2-AID-YFP (left) or GFP-AID-BubR1 (right). (C) Schematic of the protocol used to deplete endogenous p31comet and replace Mad2 or BubR1 with an AID-tagged version. Degradation of AID-tagged proteins was induced with IAA (500 μM) and cells monitored by time-lapse microscopy. (D) Immunoblot showing the levels of various proteins before and after IAA-induced degradation of (left) Mad2-AID-YFP or (right) GFP-AID-BubR1. (E–F) Time-lapse microscopy was used to determine the duration of mitosis in nocodazole (100 ng/ml) treated cells after 1) depletion of endogenous Mad2 (E, blue) or BubR1 (F, blue), 2) replacement of endogenous Mad2 or BubR1 with Mad2-AID-YFP (E, green) or GFP-AID-BubR1 (F, green) and 3) induced destruction of Mad2-AID-YFP (E, red) or GFP-AID-BubR1 (F, red). Cells were treated with nocodazole prior to filming to facilitate accumulation of the mitotic checkpoint inhibitor(s). More than 60 cells were analyzed for each condition over three independent experiments (see also Fig. S5).