Abstract
The mechanisms underlying ethylmalonic-adipic aciduria were studied in a 5-yr-old girl. Oxidation of radioactive substrates by cultured skin fibroblasts from the proband and asymptomatic family members was also determined and compared to that by normal fibroblasts and that by cells from a patient with glutaric aciduria type II.
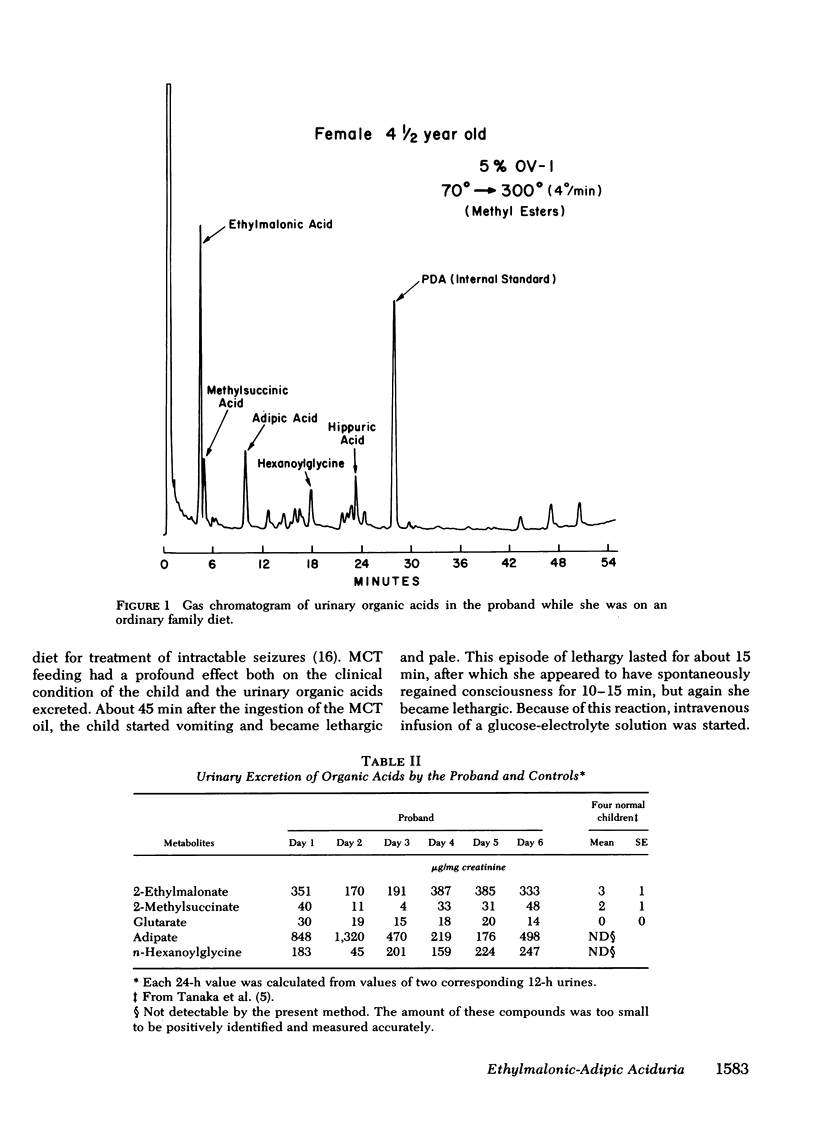
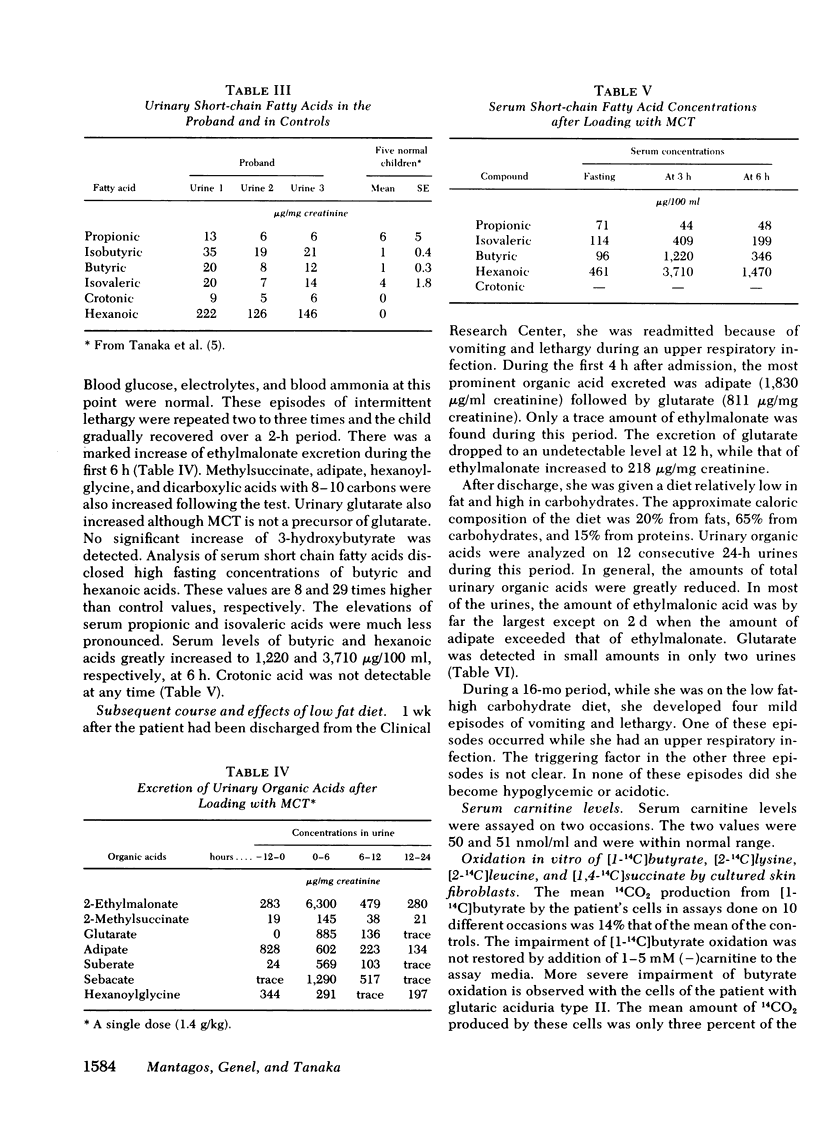
Feeding medium-chain triglycerides promptly induced vomiting and lethargy accompanied by a pronounced increase of urinary ethylmalonate. Significant increases of serum isovalerate and urinary isovalerylglycine were observed after leucine feeding, but urinary glutarate increased only slightly after lysine feeding. Thus, the results from clinical investigation remained equivocal as to whether pathways other than fatty acid oxidation were blocked in our patient.
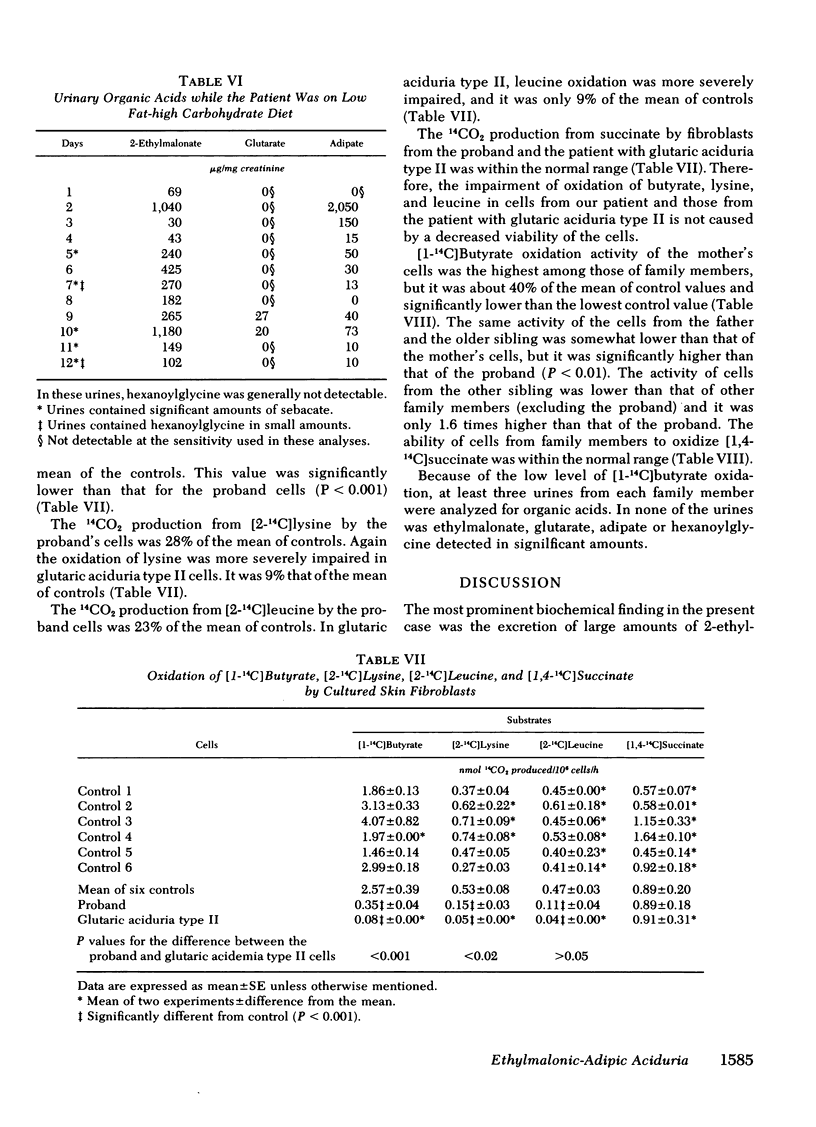
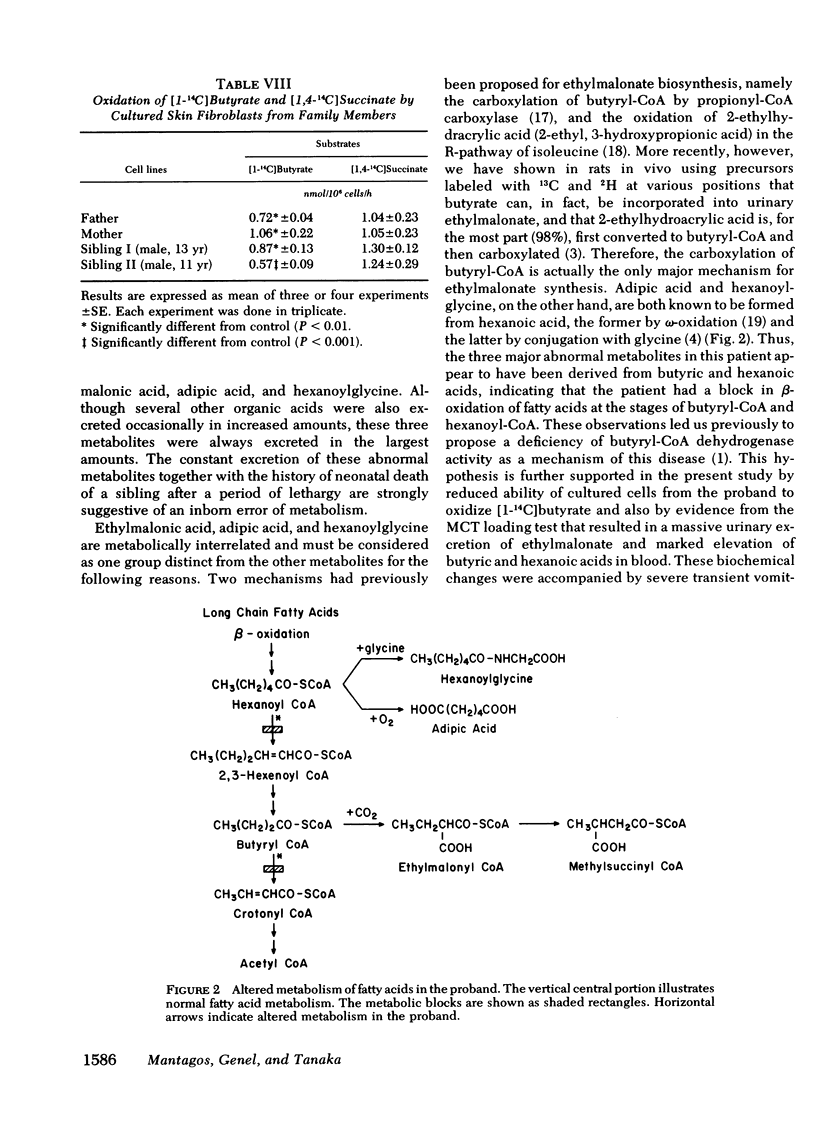
Oxidation of [1-14C]butyrate by cultured skin fibroblasts from the proband was reduced to 14% of control. In vitro oxidation of [2-14C]lysine and [2-14C]leucine was also reduced to 28 and 23% of control, respectively. Much more severe reduction in oxidation of these three substrates (3, 9, and 9%, respectively) was observed in glutaric aciduria type II cells.
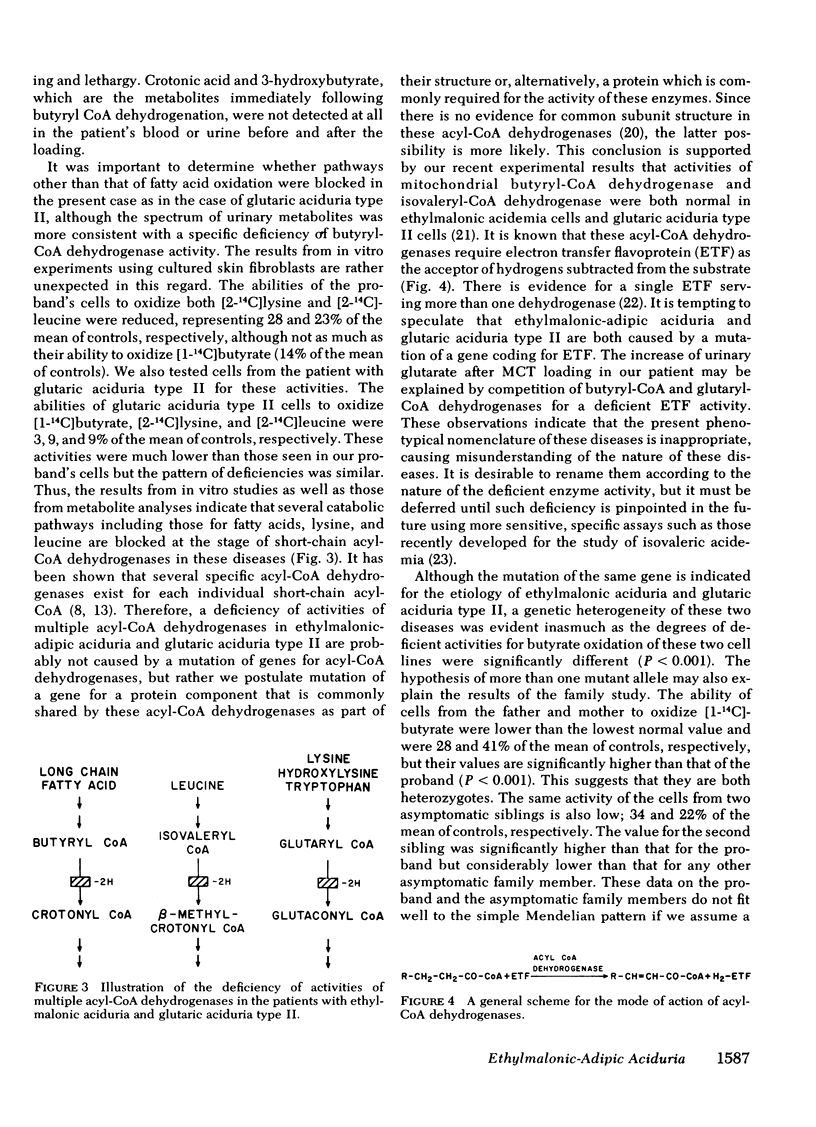
These results indicated that in the proband, degradative pathways of fatty acids, lysine, and leucine are blocked at the steps of butyryl-CoA, glutaryl-CoA, and isovaleryl-CoA dehydrogenases, respectively, as in the case of glutaric aciduria type II. Because activities of multiple acyl-CoA dehydrogenases are reduced, a deficiency of electron-transferring flavoprotein, which serves as a hydrogen-acceptor for these dehydrogenases, is postulated as the underlying mechanisms of these two diseases, but a genetic heterogeneity was indicated by significant differences in the residual activities in these two types of cells. The hypothesis of more than one mutant allele of an autosomal recessive gene was also suggested by the study on cells from asymptomatic members of the family.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baretz B. H., Lollo C. P., Tanaka K. Metabolism in rats in vivo of RS-2-methylbutyrate and n-butyrate labeled with stable isotopes at various positions. Mechanism of biosynthesis and degradation of ethylmalonyl semialdehyde and ethylmalonic acid. J Biol Chem. 1979 May 10;254(9):3468–3478. [PubMed] [Google Scholar]
- Baretz B. H., Ramsdell H. S., Tanaka K. Identification of n-hexanoylglycine in urines from two patients with Jamaican vomiting sickness. Clin Chim Acta. 1976 Nov 15;73(1):199–202. doi: 10.1016/0009-8981(76)90325-9. [DOI] [PubMed] [Google Scholar]
- Besrat A., Polan C. E., Henderson L. M. Mammalian metabolism of glutaric acid. J Biol Chem. 1969 Mar 25;244(6):1461–1467. [PubMed] [Google Scholar]
- Chappell J. B. Systems used for the transport of substrates into mitochondria. Br Med Bull. 1968 May;24(2):150–157. doi: 10.1093/oxfordjournals.bmb.a070618. [DOI] [PubMed] [Google Scholar]
- Engel A. G., Angelini C. Carnitine deficiency of human skeletal muscle with associated lipid storage myopathy: a new syndrome. Science. 1973 Mar 2;179(4076):899–902. doi: 10.1126/science.179.4076.899. [DOI] [PubMed] [Google Scholar]
- FRITZ I. B., KAPLAN E., YUE K. T. Specificity of carnitine action on fatty acid oxidation by heart muscle. Am J Physiol. 1962 Jan;202:117–121. doi: 10.1152/ajplegacy.1962.202.1.117. [DOI] [PubMed] [Google Scholar]
- Goodman S. I., Markey S. P., Moe P. G., Miles B. S., Teng C. C. Glutaric aciduria; a "new" disorder of amino acid metabolism. Biochem Med. 1975 Jan;12(1):12–21. doi: 10.1016/0006-2944(75)90091-5. [DOI] [PubMed] [Google Scholar]
- Hall C. L., Kamin H. The purification and some properties of electron transfer flavoprotein and general fatty acyl coenzyme A dehydrogenase from pig liver mitochondria. J Biol Chem. 1975 May 10;250(9):3476–3486. [PubMed] [Google Scholar]
- Hoskins D. D. The electron-transferring flavoprotein as a common intermediate in the mitochondrial oxidation of butyryl coenzyme A and sarcosine. J Biol Chem. 1966 Oct 10;241(19):4472–4479. [PubMed] [Google Scholar]
- Karpati G., Carpenter S., Engel A. G., Watters G., Allen J., Rothman S., Klassen G., Mamer O. A. The syndrome of systemic carnitine deficiency. Clinical, morphologic, biochemical, and pathophysiologic features. Neurology. 1975 Jan;25(1):16–24. doi: 10.1212/wnl.25.1.16. [DOI] [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- Mamer O. A., Tjoa S. S., Scriver C. R., Klassen G. A. Demonstration of a new mammalian isoleucine catabolic pathway yielding an Rseries of metabolites. Biochem J. 1976 Dec 15;160(3):417–426. doi: 10.1042/bj1600417a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przyrembel H., Wendel U., Becker K., Bremer H. J., Bruinvis L., Ketting D., Wadman S. K. Glutaric aciduria type II: report on a previously undescribed metabolic disorder. Clin Chim Acta. 1976 Jan 16;66(2):227–239. doi: 10.1016/0009-8981(76)90060-7. [DOI] [PubMed] [Google Scholar]
- Rétey J., Smith E. H., Zagalak B. Investigation of the mechanism of the methylmalonyl-CoA mutase reaction with the substrate analogue: ethylmalonyl-CoA. Eur J Biochem. 1978 Feb;83(2):437–451. doi: 10.1111/j.1432-1033.1978.tb12110.x. [DOI] [PubMed] [Google Scholar]
- Signore J. M. Ketogenic diet containing medium-chain triglycerides. J Am Diet Assoc. 1973 Mar;62(3):285–290. [PubMed] [Google Scholar]
- Stokke O., Goodman S. I., Thompson J. A., Miles B. S. Glutaric aciduria; presence of glutaconic and beta-hydroxyglutaric acids in urine. Biochem Med. 1975 Apr;12(4):386–391. doi: 10.1016/0006-2944(75)90071-x. [DOI] [PubMed] [Google Scholar]
- Söling H. D., Willms B., Friedrichs D., Kleineke J. Regulation of gluconeogenesis by fatty acid oxidation in isolated perfused livers of non-starved rats. Eur J Biochem. 1968 Apr;4(3):364–372. doi: 10.1111/j.1432-1033.1968.tb00220.x. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Budd M. A., Efron M. L., Isselbacher K. J. Isovaleric acidemia: a new genetic defect of leucine metabolism. Proc Natl Acad Sci U S A. 1966 Jul;56(1):236–242. doi: 10.1073/pnas.56.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J. The isolation and identification of N-isovalerylglycine from urine of patients with isovaleric acidemia. J Biol Chem. 1967 Jun 25;242(12):2966–2972. [PubMed] [Google Scholar]
- Tanaka K., Kean E. A., Johnson B. Jamaican vomiting sickness. Biochemical investigation of two cases. N Engl J Med. 1976 Aug 26;295(9):461–467. doi: 10.1056/NEJM197608262950901. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Mandell R., Shih V. E. Metabolism of [1-(14)C] and [2-(14)C] leucine in cultured skin fibroblasts from patients with isovaleric acidemia. Characterization of metabolic defects. J Clin Invest. 1976 Jul;58(1):164–172. doi: 10.1172/JCI108446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Mantagos S., Genel M., Seashore M. R., Billings B. A., Baretz B. H. New defect in fatty-acid metabolism with hypoglycemia and organic aciduria. Lancet. 1977 Nov 5;2(8045):986–987. doi: 10.1016/s0140-6736(77)90940-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K. On the mode of action of hypoglycin A. 3. Isolation and identification of cis-4-decene-1,10-dioic, cis, cis-4,7-decadiene-1,10-dioic, cis-4-octene-1,8-dioic, glutaric, and adipic acids, N-(methylenecyclopropyl)acetylglycine, and N-isovalerylglycine from urine of hypoglycin A-treated rats. J Biol Chem. 1972 Dec 10;247(23):7465–7478. [PubMed] [Google Scholar]
- Tanaka K., Ramsdell H. S., Baretz B. H., Keefe M. B., Kean E. A., Johnson B. Identification of ethylmalonic acid in urine of two patients with the vomitting sickness of Jamaica. Clin Chim Acta. 1976 May 17;69(1):105–112. doi: 10.1016/0009-8981(76)90478-2. [DOI] [PubMed] [Google Scholar]
- WAKABAYASHI K., SHIMAZONO N. Studies on omega-oxidation of fatty acids in vitro. I. Overall reaction and intermediate. Biochim Biophys Acta. 1963 Apr 23;70:132–142. doi: 10.1016/0006-3002(63)90733-9. [DOI] [PubMed] [Google Scholar]


