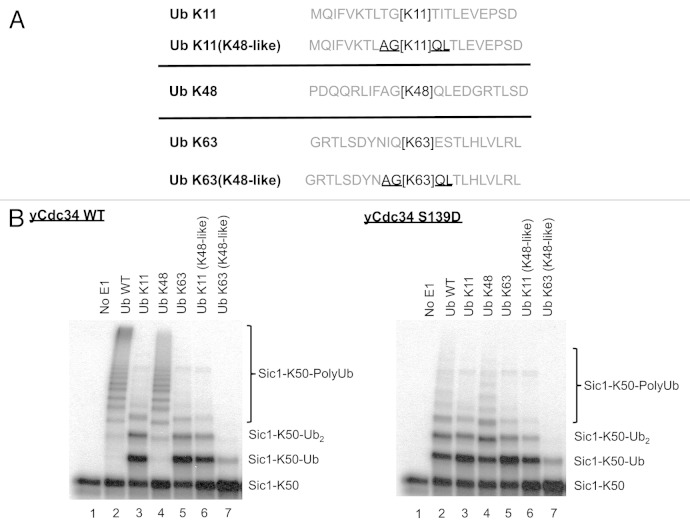
Figure 4. Mutation of residues proximal to Ub lysines 11 and 63 to those present around Ub lysine 48 does not change Ub lysine specificity of Cdc34. (A) Schematic of the sequence surrounding Ub lysines 11 (Ub K11), 48 (Ub K48) and 63 (Ub K63) encompassing amino acids from the −10 +10 relative to lysine within their wild-type context is shown. Ub lysine 11 mutant (Ub K11 (K48-like) and Ub 63 mutant (Ub K63 (K48-like) were generated where their surrounding −2, −1, +1 and +2 amino acids (underlined) were changed to those present around Ub lysine 48. (B) Cdc34 (left) and Cdc34 S139D (right) were tested for their ability to generate polyubiquitin chains in the presence of SCFCdc4, with Sic1-K50 as substrate and wild-type Ub (Ub WT), Ub K11, Ub K48, Ub K63, Ub K11(K48-like) or Ub K63(K48-like). Lane 1 is a control where E1 was omitted from the reaction.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.