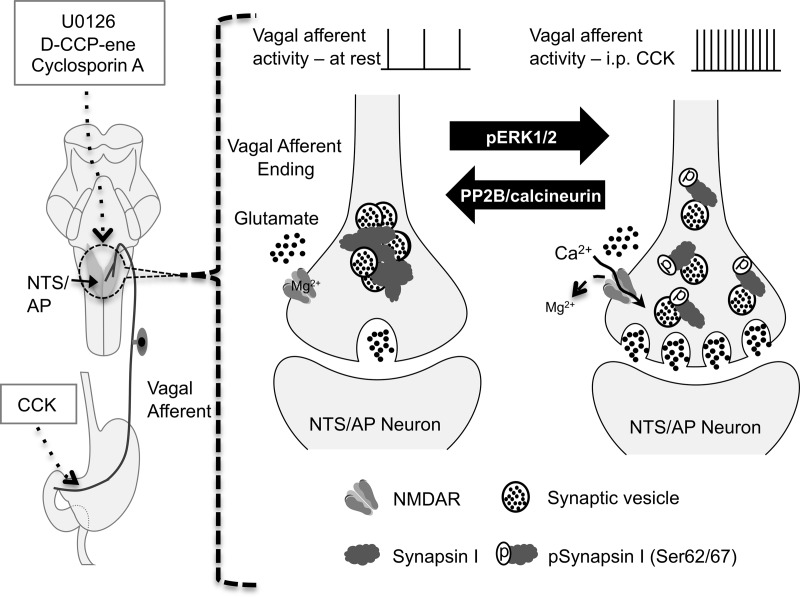
Figure 8.
Schematic model of pERK1/2-mediated synapsin I phosphorylation triggered by the activation of NMDARs on the vagal afferent endings in the NTS/AP after vagal afferent stimulation by ip CCK. Left panel, Illustration of peripheral vagal afferent endings of the stomach and small intestine, the site of action of injected CCK. Also illustrated in the left side of the diagram is the hindbrain with NTS and AP shaded. Neurons in both the NTS and AP receive synaptic input from vagal afferent endings. Note that although CCK was injected ip, all other drugs (MEK inhibitor, U0126; NMDAR antagonist, d-CPP-ene; and calcineurin inhibitor, CsA) were injected into the fourth ventricle of the hindbrain. Right panel, Illustration of synaptic vesicles within the vagal afferent endings. At rest or at low levels of stimulation, vesicles are bound to each other and to actin by synapsin I in a reserve pool. At higher rates of discharge, as evoked by CCK, terminal membrane depolarization and the presence of glutamate result in calcium entry via the NMDAR channel, resulting in the phosphorylation of ERK1/2, which phosphorylates synapsin at serines 62 and 67 (pSynapsin I). Phosphorylation of synapsin I results in the unbinding of synaptic vesicles, allowing them to enter the readily releasable pool. Increasing the size of the this pool increases synaptic strength and prolongs the period over which the terminal can sustain transmitter release. The source(s) of glutamate necessary to activate NMDAR is not known but could come from vagal afferents themselves, axoaxonal synapses on vagal afferents, or glia.