Abstract
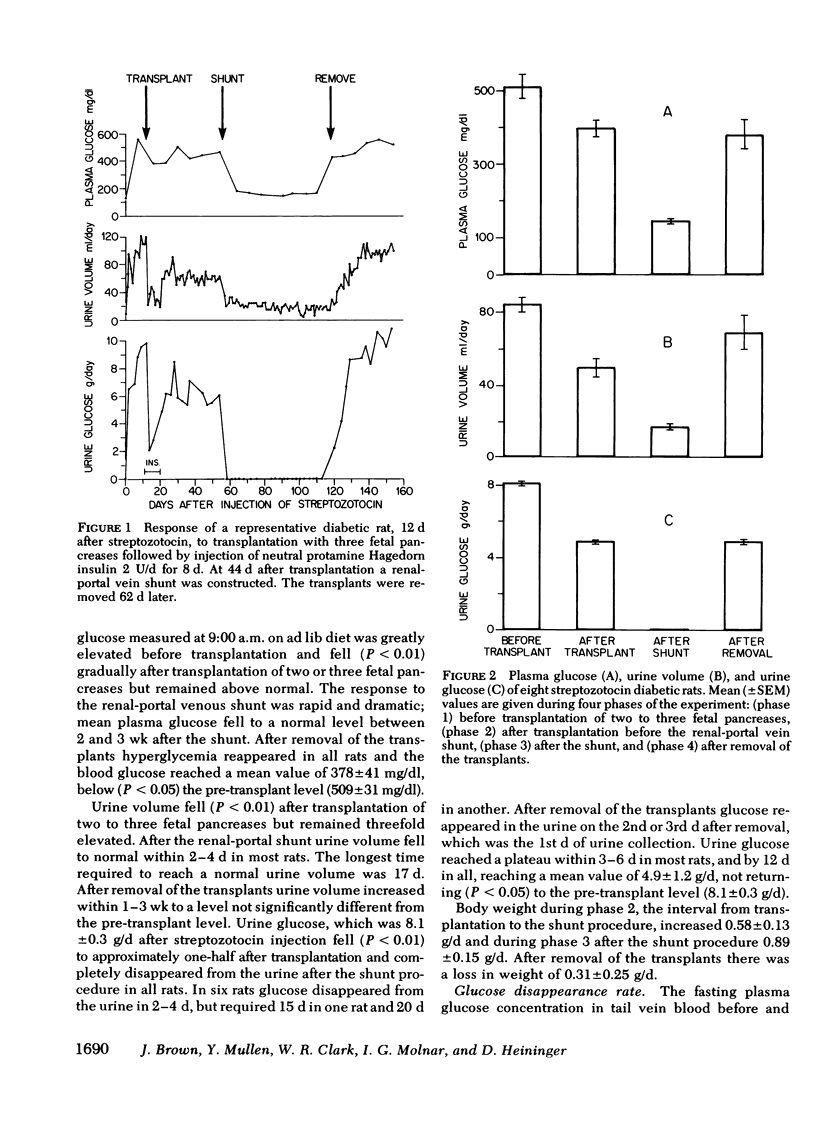
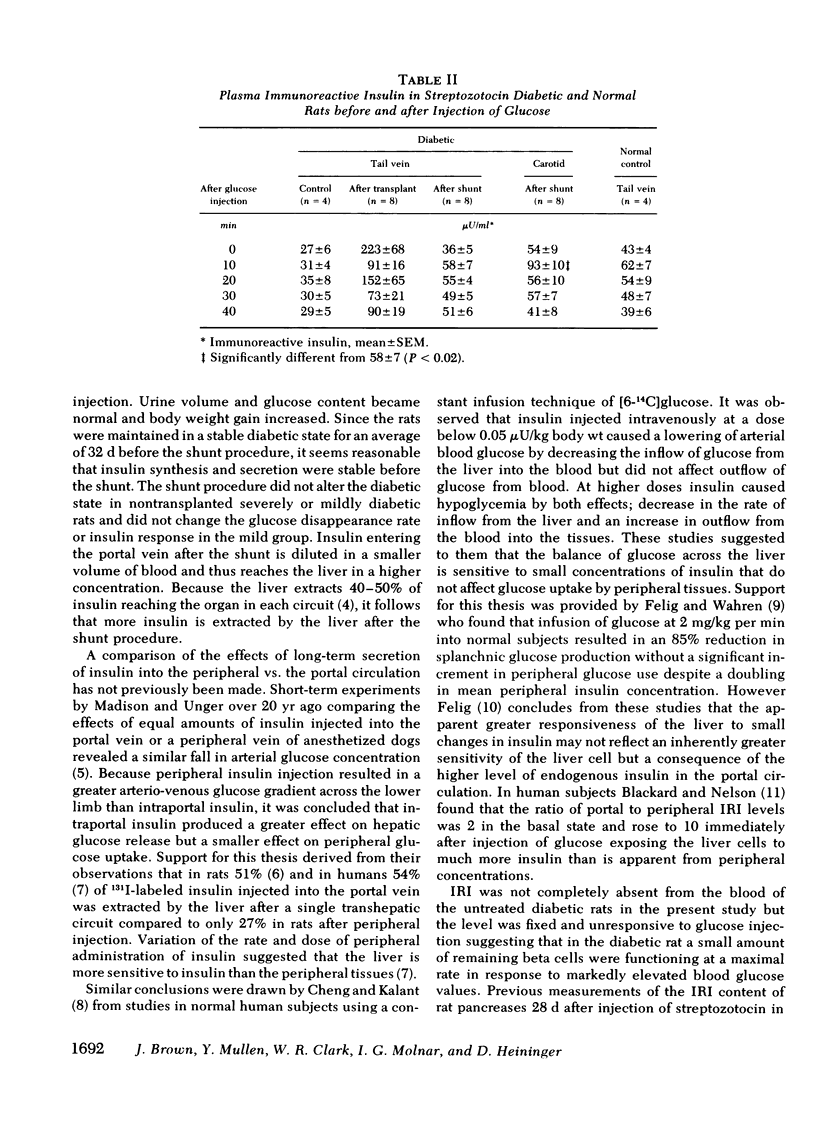
The importance of the hepatic portal circulation in the response to insulin was assessed in streptozotocin-diabetic rats transplanted with syngeneic fetal pancreases. Partial reversal of diabetes was accomplished by transplantation of two or three fetal pancreases beneath the capsule of the kidney; complete reversal followed shunting of the venous drainage from the transplants to the liver. Plasma glucose after streptozotocin of 509±31 mg/dl (mean±SEM) fell after transplantation to 395±23 and after the shunt to 143±5 mg/dl. Urine volume fell from 84±4 to 50±5 ml/d and then to normal (17±1 ml/d) after the shunt. Glucose excretion which was 8.1±0.3 g/d after streptozotocin fell after transplantation to 4.8±0.3 g/d and after the shunt completely disappeared from the urine. The disappearance rate of glucose injected into the circulation, which was 0.50±0.07%/min in untreated diabetes, increased to 1.39±0.38%/min after transplantation and to 2.52±0.31%/min after the shunt, not different from normal controls (2.79±0.25). Plasma immunoreactive insulin (IRI) was below normal (25-35 μU/ml) and unresponsive to glucose in untreated diabetic rats. After transplantation IRI levels ranged from 73-223 μU/ml and there was no rise after glucose injection. After the shunt both the basal IRI (36±5 μU/ml) and the peak response to glucose at 10 min (58±7 μU/ml) were the same as in normal controls (42±4 and 62±7 μU/ml, respectively). The fall in IRI after the shunt is explained by increased extraction of insulin passing into the liver and also diminished secretion. After removal of the transplants plasma glucose and urine values returned almost to pretransplant levels.
Secretion of insulin by transplanted pancreases into the liver enhances the effectiveness probably by increased extraction and action and reveals the importance of the normal route for insulin delivery.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackard W. G., Nelson N. C. Portal and peripheral vein immunoreactive insulin concentrations before and after glucose infusion. Diabetes. 1970 May;19(5):302–306. doi: 10.2337/diab.19.5.302. [DOI] [PubMed] [Google Scholar]
- Brown J., Clark W. R., Molnar I. G., Mullen Y. S. Fetal pancreas transplantation for reversal of streptozotocin-induced diabetes in rats. Diabetes. 1976 Jan;25(1):56–64. doi: 10.2337/diab.25.1.56. [DOI] [PubMed] [Google Scholar]
- Brown J., Molnar I. G., Clark W., Mullen Y. Control of experimental diabetes mellitus in rats by transplantation of fetal pancreases. Science. 1974 Jun 28;184(4144):1377–1379. doi: 10.1126/science.184.4144.1377. [DOI] [PubMed] [Google Scholar]
- Bucolo R. J. Obesity, hyperinsulinemia, and portal blood flow. A theoretic study of the effects of increased portal blood flow upon fasting peripheral insulin concentrations. Diabetes. 1978 Aug;27(8):840–848. doi: 10.2337/diab.27.8.840. [DOI] [PubMed] [Google Scholar]
- Cheng J. S., Kalant N. Effects of insulin and growth hormone on the flux rates of plasma glucose and plasma free fatty acids in man. J Clin Endocrinol Metab. 1970 Dec;31(6):647–653. doi: 10.1210/jcem-31-6-647. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J. Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. J Clin Invest. 1971 Aug;50(8):1702–1711. doi: 10.1172/JCI106659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junod A., Lambert A. E., Stauffacher W., Renold A. E. Diabetogenic action of streptozotocin: relationship of dose to metabolic response. J Clin Invest. 1969 Nov;48(11):2129–2139. doi: 10.1172/JCI106180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. D., Foy J. M. Effects of experimental diabetes and genetic obesity on regional blood flow in the rat. Diabetes. 1977 Aug;26(8):786–792. doi: 10.2337/diab.26.8.786. [DOI] [PubMed] [Google Scholar]
- MADISON L. L., COMBES B., STRICKLAND W., UNGER R., ADAMS R. Evidence for a direct effect of insulin on hepatic glucose output. Metabolism. 1959 Jul 2;8(4 Pt 2):469–471. [PubMed] [Google Scholar]
- MADISON L. L., UNGER R. H. The physiologic significance of the secretion of endogenous insulin into the portal circulation. I. Comparison of the effects of glucagon-free insulin adminstered via the portal vein and via a peripheral vein on the magnitude of hypoglycemia and peripheral glucose utilization. J Clin Invest. 1958 May;37(5):631–639. doi: 10.1172/JCI103646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy R. C., Hegre O. D. Syngeneic transplantation of fetal rat pancreas. III. Effect of insulin treatment on the growth and differentiation of the pancreatic implants after reversal of diabetes. Diabetes. 1979 Feb;28(2):141–146. doi: 10.2337/diab.28.2.141. [DOI] [PubMed] [Google Scholar]
- Misbin R. I., Merimee T. J., Lowenstein J. M. Insulin removal by isolated perfused rat liver. Am J Physiol. 1976 Jan;230(1):171–177. doi: 10.1152/ajplegacy.1976.230.1.171. [DOI] [PubMed] [Google Scholar]
- Mullen Y. S., Clark W. R., Molnar I. G., Brown J. Complete reversal of experimental diabetes mellitus in rats by a single fetal pancreas. Science. 1977 Jan 7;195(4273):68–70. doi: 10.1126/science.137524. [DOI] [PubMed] [Google Scholar]
- Pipeleers D. G., Pipeleers-Marichal M. A., Karl I. E., Kipnis D. M. Secretory capability of islets transplanted intraportally in the diabetic rat. Diabetes. 1978 Aug;27(8):817–824. doi: 10.2337/diab.27.8.817. [DOI] [PubMed] [Google Scholar]
- Shah J. H., Wongsurawat N., Aran P. P., Motto G. S., Bowser E. N. A method for studying acute insulin secretion and glucose tolerance in unanesthetized and unrestrained rats. The effect of mild stress on carbohydrate metabolism. Diabetes. 1977 Jan;26(1):1–6. doi: 10.2337/diab.26.1.1. [DOI] [PubMed] [Google Scholar]


