Abstract
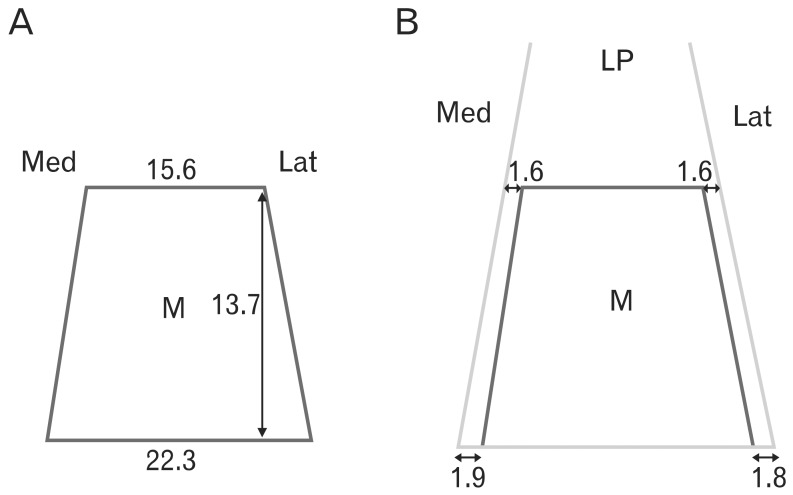
Eyelid anatomy, including thickness measurements, was examined in numerous age groups. The thickest part of the upper eyelid is just below the eyebrow (1.127±238 µm), and the thinnest near the ciliary margin (320±49 µm). The thickness of skin at 7 mm above the eyelashes was 860±305 µm. The results revealed no significant differences among the age groups. Fast fibers (87.8±3.7%) occupied a significantly larger portion of the orbicularis oculi muscle (OOM) than nonfast fibers (12.2±3.7%). The frontalis muscle passed through and was inserted into the bundles of the OOM on the superior border of the eyebrow at the middle and medial portions of the upper eyelid. Laterally, the frontalis muscle inserted about 0.5 cm below the superior border of the eyebrow. Fast fibers occupied a significantly larger portion of the OOM than did non-fast fibers. The oculomotor nerve ends that extend forward to the distal third of the levator muscle are exposed and vulnerable to local anesthetics and may be numbed during blepharoplasty. The orbital septum consists of 2 layers. The outer layer of loose connective tissue descends to interdigitate with the levator aponeurosis and disperses inferiorly. The inner layer follows the outer layer, then reflects and continues posteriorly with the levator sheath. Widths of the tarsal plate at its lower border, mid-height, and upper border were 21.8±1.8, 16.2±1.6, and 8.3±1.0 mm, respectively. The widths of the levator aponeurosis were 32.0±2.2, 29.2±3.5, and 27.2±3.9 mm, respectively. Below the levator, the "conjoint fascial sheath" (CFS) is attached to the conjunctival fornix. The CFS was 12.2±2.0 mm anteroposterior length and 1.1±0.1 mm thick. The shape was equilateral trapezoid with a longer base anteriorly. The superior palpebral muscle was trapezoidal. The lengths of its sides were 15.58±1.82 and 22.30±5.25 mm, and its height was 13.70±2.74 mm. The width of the levator aponeurosis was approximately 4 mm wider than the superior palpebral muscle.
Keywords: Eyelids, Anatomy and histology, Blepharoplasty, Blepharoptosis
Introduction
A thorough understanding of the anatomy of the upper eyelid and surrounding structures is mandatory to achieve satisfactory surgical results and avoid potential complications. The aim of this review is to familiarize the reader with critical upper eyelid anatomy relating to upper blepharoplasty or blepharoptosis surgery.
Thickness of the Upper Eyelid in a Korean Population
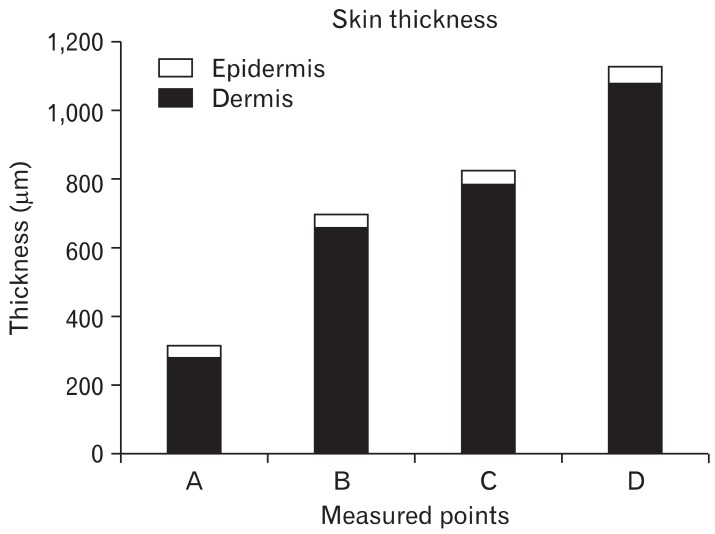
Few reports have described the thickness of upper eyelid skin in Koreans. Barker [1] measured upper eyelid thickness as 330-355 µm in 4 Caucasian cadavers. The average thickness of upper eyelid skin in Korean individuals is 521±115.8 µm [2]. Hwang et al. [3] found that upper eyelid skin thickness varied among the 5 levels at which measurements were taken. The thickest part of the upper eyelid is just below the eyebrow (1,127±238 µm), and the thinnest skin is near the ciliary margin (320±49 µm). The thickness of the upper tarsal area and mid-tarsal area are 832±213 µm and 703±103 µm, respectively. The epidermis accounted for 11.2% of entire skin thickness near the ciliary margin. However, the epidermis represented a smaller proportion (4.2-5.5%) of overall skin thickness at other levels (P=0.000). The distance between the ciliary margin and the point of this sharp increase in thickness was 1.89±0.23 mm. The length of the tarsal plate was 8.88±0.81 mm (Fig. 1). These results show that upper eyelid skin is thicker in Korean than in Caucasian individuals [3].
Fig. 1.
Histograms showing epidermis and dermis thickness as measured at various points. A, near the ciliary margin; B, midtarsus; C, upper tarsal border; D, lower border of the eyebrow.
Does Upper Eyelid Skin Thin with Age?
Gonzalez-Ulloa and Flores [4] reported that skin thickness substantially diminishes by 60 years of age. A hollow or depression develops in the temporal and buccal regions because less fat is present. However, upper eyelid skin may not thin with age. Hykin and Bron [5] measured lid margin thickness and found that cutaneous hyperkeratinization of the lid margin increased with age.
Hwang et al. [6] measured the thickness of upper eyelid skin 7 mm above the eyelashes in 61 Korean women. Thickness varied from 818±85 µm in subjects aged 60 years or older to 884±112 µm in subjects who were 21-30 years old; the mean was 860±305 µm. Thickness of the epidermis varied from 46±6 µm in subjects who were 41-50 years old as compared to 52±10 µm in subjects aged 31-40 years; the mean was 49±9 µm. There were no significant differences among the age groups (P=0.440). Thickness of the dermis varied from 771±78 µm in subjects aged more than 61 years to 834±112 µm in subjects who were 21-30 years old; the mean was 811±117 µm. There were no significant differences between the age groups (P=0.553) (Table 1). It is notable that upper eyelid skin thickness did not correlate with age [6].
Table 1.
Thickness of skin in different age groups
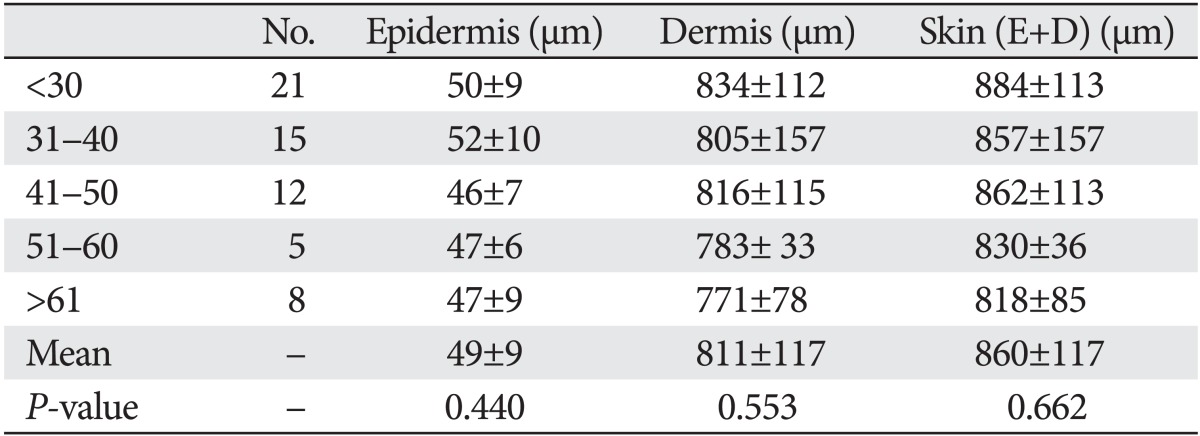
Reproduced from Hwang K et al. J Craniofac Surg 2006;17:474-6 [6], with permission from Wolters Kluwer and Lippincott Williams & Wilkins.
Muscle Fiber Types Present in the Human Orbicularis Oculi Muscle (OOM)
The OOM is a complex facial muscle involved in eyelid closure. The OOM consists of palpebral, pre-septal, pre-tarsal, and ciliary (muscle of Riolan) parts [7]. It is expected that each part of the OOM comprises various types of muscle fibers because form follows function [8].
McLoon and Wirtschafter [9] compared muscle fiber types of the pre-tarsal and pre-septal parts of the OOM in rabbits and monkeys. He reported that the pre-tarsal portion of the muscle, that closest to the eyelid margin, is almost completely composed of type II fibers. Type II fibers also predominate in the pre-septal portion of the muscle, but 10-20% of muscle fibers in this region were type I fibers. With regard to measurements of the pre-septal lid, our measurements of upper eyelid thickness in humans (88%) were very similar to lower-eyelid measurements obtained in cynomolgus monkeys. The pre-tarsal portion of the human upper eyelid had a high proportion of fast fibers (87%), similar to the lower eyelid of the monkey (100%).
Goodmurphy and Ovalle [10] compared muscle fiber composition in the palpebral part of the OOM and corrugator in humans. He obtained samples from 14 patients who had undergone cosmetic blepharoplasties. The OOM fibers in these patients were small and rounded, 89% were of the fast-twitch (type II). In the corrugator, only 49% of the fibers were of the fast-twitch variety [10].
Hwang et al. [11] recently used immunohistochemistry to determine the number and size of muscle fibers in the pre-septal, pre-tarsal, and ciliary parts of the human orbicularis oculi muscle. Fast fibers (mean, 87.8±3.7%; range, 85.6-91.7%) occupied a significantly larger portion of the muscle (P=0.000, t-test) than non-fast fibers (mean, 12.2±3.7%; range, 8.3-14.4%). In comparison to the pre-septal and pre-tarsal portion of the eyelid, the ciliary part had a significantly (P=0.019, Scheffé) higher proportion (91.7%) of fast fibers than the pre-tarsal portion (86.6%). The diameter of the fast fibers (mean, 17.7±2.6 µm) was significantly greater (P=0.000, t-test) than that of the non-fast fibers (mean, 13.0±2.1 µm) [11]. Hwang et al.'s results [12] showed that the eyelid has a higher proportion of fast muscle fibers than the mouth (pars peripheralis, 73% fast fibers; pars marginalis, 66% fast fibers). The results Hwang et al. [12] obtained for the pre-septal (88%) and pre-tarsal (87%) areas are in agreement with those reported by Goodmurphy and Ovalle (89%) [10], based on his measurements of the palpebral portion (89%) (Table 2).
Table 2.
Percent of fast fibers of facial muscles in the literature
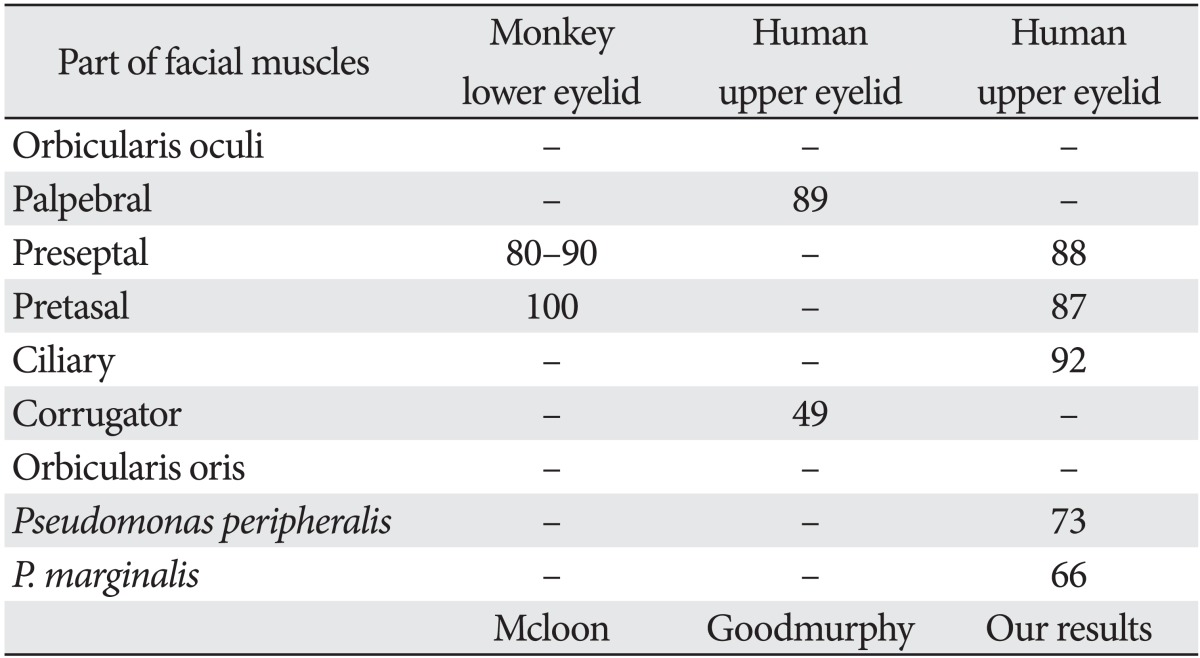
Reproduced from Hwang K et al. J Craniofac Surg 2011;22:1827-30 [11], with permission from Wolters Kluwer and Lippincott Williams & Wilkins.
Values are presented as percentage.
Frontalis Muscle Insertion
In 'Gray's Anatomy,' the frontalis muscle is described as a thin and quadrilateral muscle that adheres to the superficial fascia. It has no bony attachments. Its medial fibers are continuous with those of the procerus; its intermediate fibers blend with the corrugator supercilii and orbicularis oculi; and its lateral fibers blend with the latter muscle as it passes over the zygomatic process of the frontal bone [13].
Ramirez and Peña [14] insisted that a clear surgical plane could be developed to separate the OOM from the frontalis muscle. Knize [15] reported that the frontalis penetrated the surface of the deep orbicularis oculi muscle. Knize [15] also reported that no plane existed between the OOM and the skin. A septal fiber extending from the frontalis muscle to the skin was not constant in all of the study subjects.
Karacalar et al. [16] found that the fibers of the frontalis muscle usually intermingled with the fibers of the orbicularis muscle. These fibers entered the posterior and/or anterior surfaces of the OOM and traveled within the same plane throughout their course, completely enveloping the fibers of the OOM [16].
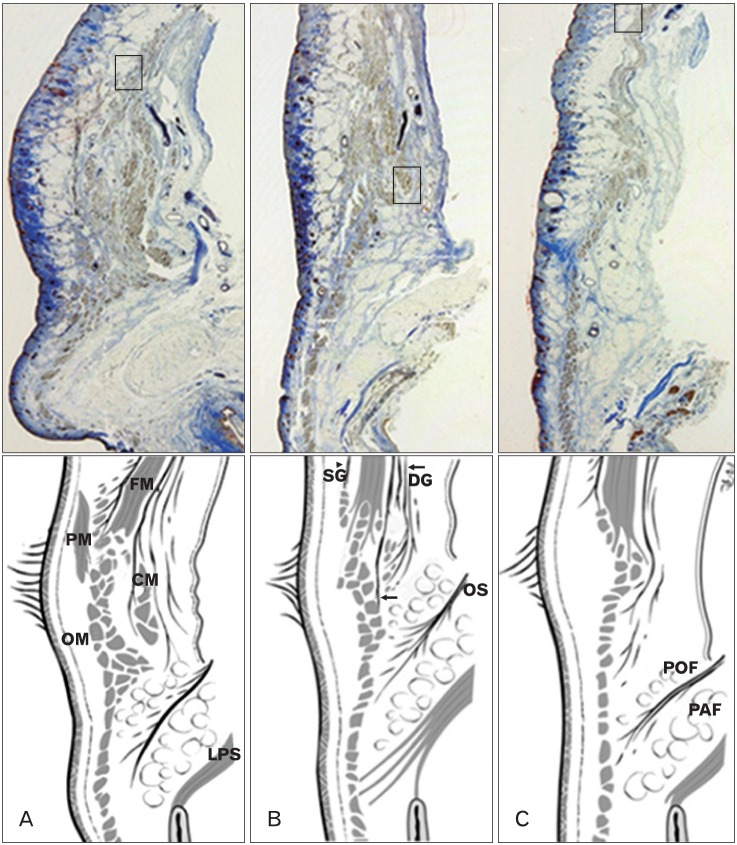
Hwang et al. [17] reported that the frontalis muscle passed through and inserted into the bundles of the OOM on the superior border of the eyebrow at the middle and medial sides of the upper eyelid (Fig. 2). On the lateral side, it inserted about 0.5 cm below the superior border of the eyebrow. On the medial side of the eyelid, the most distal frontalis muscle was located deep to the procerus muscle and superficial to the corrugator muscle [17].
Fig. 2.
Sagittal sections of the upper eyelid. The anterior surface of the frontalis muscle is enveloped by superficial galea (SG, arrowhead) and deep galea (DG, arrows) on its posterior surface. The frontalis muscle (FM) passes through and inserts in the bundles of the orbicularis oculi muscle (OM). (A) Medial section. The FM inserts in the superior border of the eyebrow. The most distal part of the FM is located deep to the procerus muscle (PM) and superficial to the corrugator muscle (CM). (B) Middle section. The FM inserts in the superior border of the eyebrow. (C) Lateral section (the FM inserts about 0.5 cm below the superior border of the eyebrow). OS, orbital septum; PAF, pre-aponeurotic fat; POF, pre-orbicularis fascia.
Oculomotor Nerve Distribution to the Levator Palpebrae Superioris Muscle
Transient diplopia or blepharoptosis occurs commonly when upper eyelid surgery has been performed with the patient under local anesthesia. Rainin and Carlson [18] hypothesized that some cases of postoperative diplopia and blepharoptosis could be attributed to the myotoxic effects of local anesthetics on the extraocular or levator muscles. Kalichman [19] suggested that lidocaine may exert neurotoxic effects.
Hwang et al. [20] found that the nerve branches of the superior division of the oculomotor nerve innervated the proximal third (type I) in 2 of 30 levator palpebrae superioris muscles (6.7%), extended to the middle third (type II) in 8 of 30 muscles (26.7%), and reached the distal third (type III) in 20 of 30 muscles (66.7%). The terminal branches ran through the medial third (type IIIa) in 6 of 20 type III levator palpebrae superioris muscles (30%), the central third (type IIIb) in 8 muscles (40%), and the lateral third (type IIIc) in 6 muscles (30%). The oculomotor nerve ends that extend forward to the distal third of the levator palpebrae superioris muscle (type III) are exposed and vulnerable to local anesthetics and may be numbed during blepharoplasty [20].
Anatomy at the Junction of the Orbital Septum and Levator Aponeurosis in an Asian Population
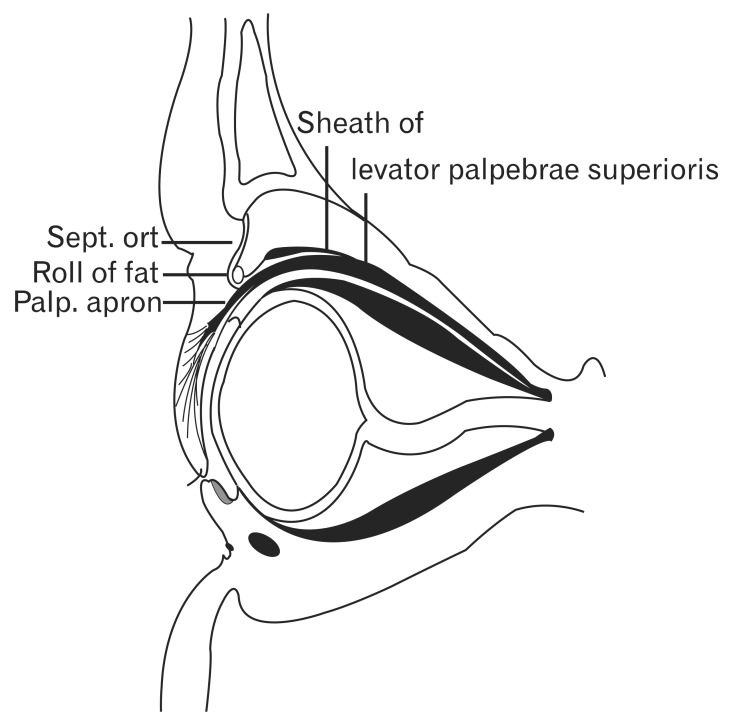
By looking at vertical sections of the orbit, Whitnall [21] observed the superficial part of the levator sheath to form a conspicuous band above the levator palpebrae muscle just behind the aponeurosis. The levator sheath can be traced anteriorly as a delicate layer of connective tissue running over the aponeurosis and posterior to the orbital septum, ending at the periosteum of the supraorbital bony rim just posterior to the origin of the orbital septum (Fig. 3) [21].
Fig. 3.
Schema of a sagittal section of the eyelid. Reproduced from Whitnall SE. J Anat Physiol 1911;45:131-9 [21], with permission from John Wiley & Sons Ltd. Sept. ort, septum orbitale; Palp. aporn, palpebral aponeurosis.
Hwang et al. [22] confirmed that the orbital septum originates from the arcus marginalis of the frontal bone and consists of 2 layers. The whitish outer (superficial) layer, containing vessels running vertically, descends just inside the OOM to interdigitate with the levator aponeurosis via loose connective tissue, and then disperses inferiorly. The inner (deep) layer follows the superficial one initially, then changes direction at the levator aponeurosis and continues posteriorly with the levator sheath [22].
Width of the Levator Aponeurosis vs. Tarsal Plate
Wolff reported that the tendinous extensions of the aponeurosis pass through the muscular bundles of the orbicularis, to insert into the skin. Such an insertion would explain the location of the superior lid crease, or sulcus, a lid feature with substantial aesthetic significance [23].
Werb's studies [24] have suggested that the aponeurosis is firmly attached to the fibrous covering on the deep surface of the orbicularis, but does not provide tendinous extensions into the skin. 'Gray's Anatomy' stated that the lateral horn is much more strongly developed and-in the form of a conspicuous ligament, 3-4 mm broad-cuts into and subdivides the lacrimal gland, which is, as it were, folded around it. Below the gland, the lateral horn is inserted into the orbital tubercle of the zygomatic bone, covering the insertion of the true lateral ligament of the tarsal plates. On the medial side, the aponeurosis abruptly becomes less tendinous as it passes over and comes into close contact with the reflected tendon of the superior oblique muscle. The opposing surfaces are smooth and shining, but no definite bursal formation can be identified. From this point, the medial horn turns downwards at an angle and can be traced only with difficulty, because it exists as loose strands that fuse with the medial palpebral ligament, thus reaching the bone [13].
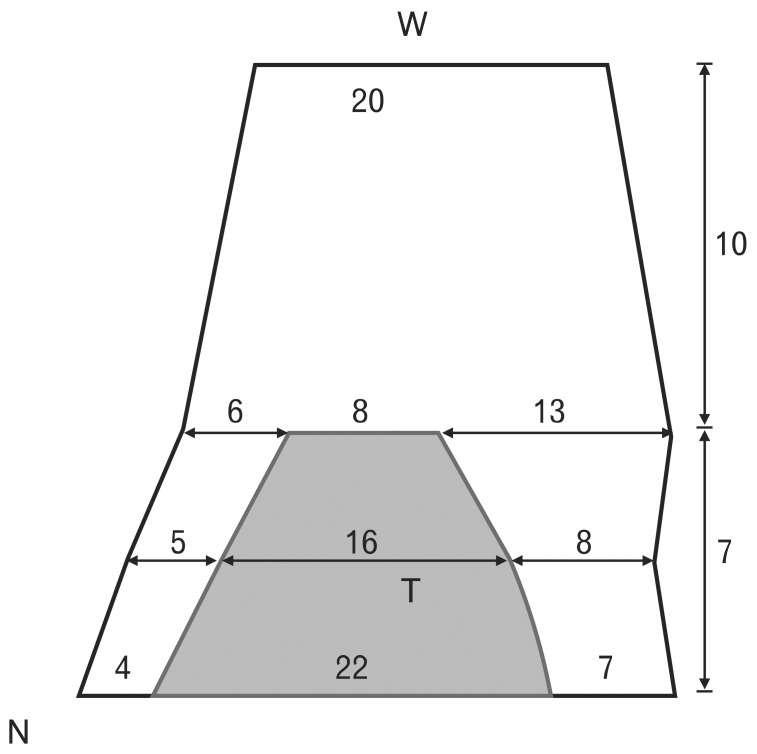
Hwang et al. [25] measured the width of the tarsal plate at its lower border, mid-region, and upper border (21.8±1.8, 16.2±1.6, and 8.3±1.0 mm, respectively). The widths of the levator aponeurosis at the lower border, mid-region, and upper border of the tarsal plate were 32.0±2.2, 29.2±3.5, and 27.2±3.9 mm, respectively. Tarsal plate width was 19.9±4.3 mm at the anterior border of the superior transverse ligament. The width of the levator aponeurosis was broader than that of the tarsal plate at all 3 levels. The medial brims of the levator aponeurosis at the lower border, mid-region, and upper border of the tarsal plate were 3.6±1.1, 5.1±1.0, and 6.2±1.1 mm, respectively. The lateral brims of the levator aponeurosis at the lower border, mid-region, and upper border of the tarsal plate were 6.6±0.9, 7.9±2.6, and 12.7±3.7 mm, respectively (Fig. 4) [25].
Fig. 4.
Schema of the levator aponeurosis (black trapezoid) and tarsal plate (T, gray trapezoid). Upper: Levator aponeurosis width was measured at the lower border, mid-region, and upper border of the tarsal plate to be 32.0±2.2 mm, 29.2±3.5 mm, 27.2±3.9 mm, respectively. Lower: The medial brim of the levator aponeurosis at the lower border, mid-region, and upper border of the tarsal plate was 3.6±1.1 mm, 5.1±1.0 mm, and 6.2±1.1 mm, respectively. The lateral brim of the levator aponeurosis at the lower border, mid-region, and upper border of the tarsal plate was 6.6±0.9 mm, 7.9±2.6 mm, and 12.7±3.7 mm, respectively. N, nasal side; W, superior transverse ligament of Whitnall.
Attachment of the Levator-Superior Rectus Fascial Sheath to the Conjunctival Fornix
Numerous reports have described the structure lying between the levator and the superior rectus. In 1874, Merkel termed it the "check ligament (fascien zipfel)" [21]. In 1886, Lockwood [26] described it as mutual adhesion of the levator and superior rectus. In 1887, Motais called it the superior check ligament; he believed the structure to represent expansions of the sheath of the superior rectus [21]. In 1904, Toldt et al. [27] proposed naming the structure Fascienzipfel, which denotes fibrous slips of the levator-superior rectus fascial sheath, or, more concisely, check ligaments. In 1914, Whitnall [28] pointed out that the levator and the underlying superior rectus muscles are intimately connected through fusion of their fascial sheaths. Anteriorly, where the 2 muscles separate to reach their respective insertions, the fascia between them forms a thick mass, which is fixed to the superior conjunctival fornix. This indirect fascial attachment, which is shared with the superior rectus, is described as an additional insertion of the levator palpebrae superioris [28]. In 1932, Whitnall [29] again addressed the common mass of tissue filling up the angle of divergence between the levator and superior rectus. In his figure, he referred to this structure as the "conjoint fascial sheath of the levator and superior rectus attached to the conjunctival fornix." Whitnall [29] insisted that the structure should be described as an additional insertion of the levator as well as the superior rectus.
In 1957, Fink [30] named this structure the transverse superior fascial expansion and considered it to contribute to check action. In 1986, Manson [31] published his support of Whitnall's description. His research had convinced him that a conjoined thickening of the fascia from the superior rectus and levator muscles extends to reach the conjunctiva and Tenon's capsule. Manson [31] described the structure as "combined sheaths of levator and superior rectus'' and a "combined muscle sheath." In 1996, Ettl et al. [32] reported that Whitnall's ligament could be considered to consist of 2 distinct parts: the transverse superior fascial expansion inferior to the levator and the superior transverse ligament superior to the levator.
Holmström et al. [33, 34] proved that the check (or suspensory) ligament of the superior fornix of the conjunctival sac consists of a plate of connective tissue emanating from the ligament that attaches to the conjunctiva at the level of the superior fornix, thereby stabilizing it.
Hwang et al. [35] recently reported that the superior rectus is covered by a thick, fibrous sheath (Fig. 5). In Whitnall's description, he called the thickened portion the "conjoint fascial sheath" (CFS) of the levator and superior rectus that attached to the conjunctival fornix. The CFS is located 2.5±0.2 mm (range, 2 to 8 mm) posterior to the fornix. The anteroposterior length was 12.2±2.0 mm (range, 8-14 mm) thickness was 1.1±0.1 mm (range, 0.5-1.5 mm). The structure is shaped like an equilateral trapezoid with a long anterior base. Posteriorly, it extended from the fascia of the levator and superior rectus. Anteriorly, superficial and deep extensions of the CFS continued approximately 2 mm to the superior conjunctival fornix and then 2-3 mm distally along and beneath the palpebral and bulbar conjunctiva [35].
Fig. 5.
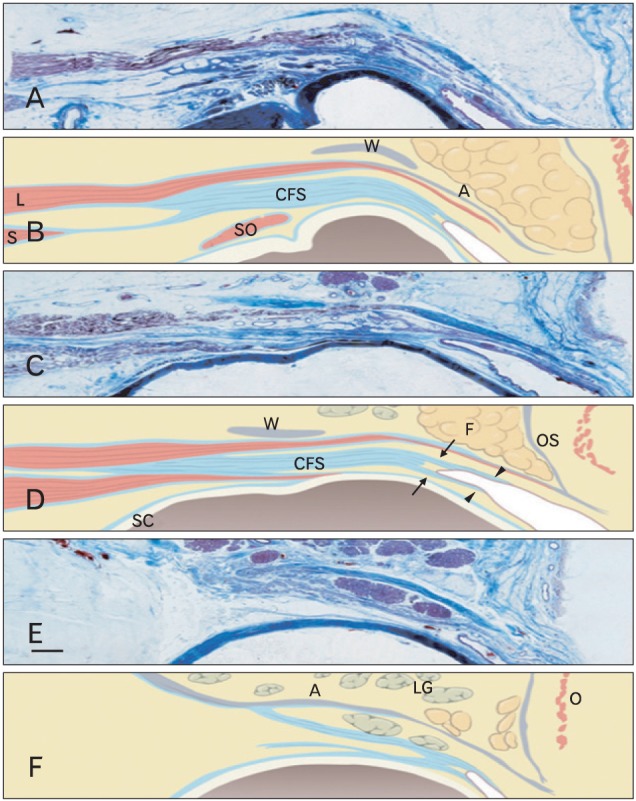
Microscopic findings of the upper eyelid. (A, B) Through the medial limbus line, (C, D) midpupillary line, (E, F) through the lateral limbus line. The conjoint fascial sheath (CFS) was located in the intermuscular space between the anterior third of the superior rectus (S) and the levator (L). Posteriorly, it extended from the fascia of the levator and superior rectus. Anteriorly, superficial and deep extensions (arrows) of CFS continued approximately 2 mm to the superior conjunctival fornix and then 2-3 mm distally (arrowheads) along and beneath the palpebral and bulbar conjunctiva. A, levator aponeurosis; F, pre-aponeurotic fat; LG, lacrimal gland; O, orbicularis oculi muscle; OS, orbital septum; SO, superior oblique; W, Whitnall's superior transverse ligament. Scale bar in (E)=2 mm (A-F).
Size of the Superior Palpebral Involuntary Muscle (Müller's Muscle)
Wolff 's anatomy described the superior palpebral muscle as 15-20 mm wide at its site of origin [23]. Whitnall [29] reported that the smooth muscle fibers arise from the striated fibers of the levator as elastic tendons. Duke-Elder and Wybar [36] also reported striated muscle fibers to originate in elastic tendon tissue. This close association of smooth with striated muscle is particularly clear in human anatomy. Wolff maintains that Muller's muscle originates among the fibers of the levator muscle, just behind the fornix [36].
Isaksson [37] described the smooth and striated muscle fibers lying interwoven in the terminal interstitia of the levator muscle and said that the smooth muscle fibers at this junction lie largely in transverse bundles, although these may have a highly variable course. Peripheral to the junction, these fibers become longitudinal.
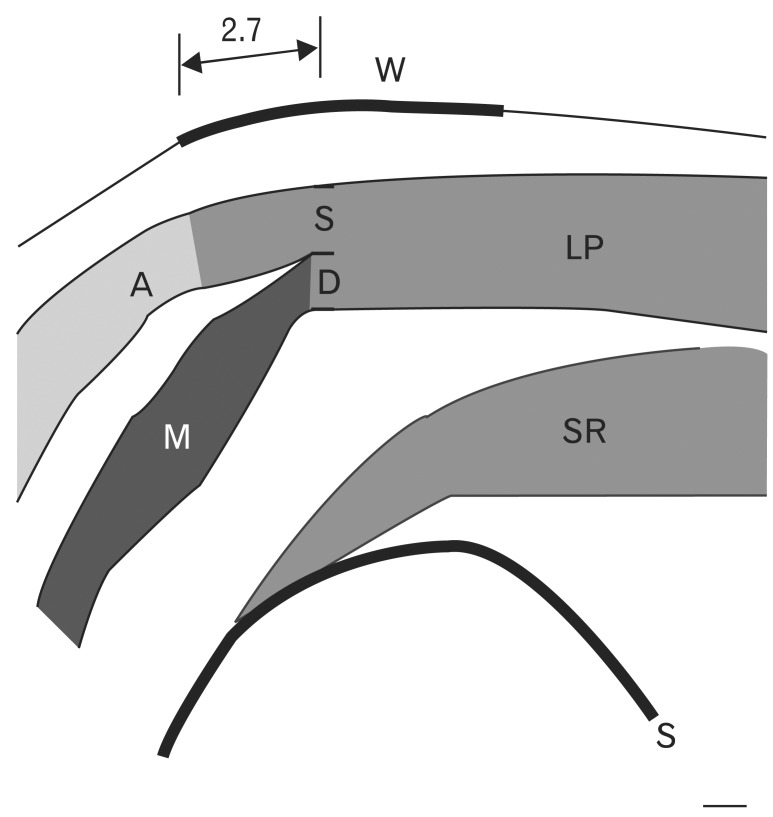
Hwang et al. [38] observed that the levator palpebrae superioris muscle was divided into superficial and deep parts below the superior transverse ligament (Fig. 6). The levator aponeurosis originates from the superficial part, and the superior palpebral muscle originates from the deep part of the levator palpebrae superioris muscle. The aponeurosis inserts into the upper border of the tarsus. The superior palpebral muscle fibers rise 2.71±0.64 mm posterior to the anterior border of the superior transverse ligament. The superior palpebral muscle is trapezoidal, with sides of length 15.58±1.82 and 22.30±5.25 mm, and height of 13.70±2.74 mm. The levator aponeurosis covered the superior palpebral muscle anteriorly. The width of the levator aponeurosis was approximately 4 mm wider than the superior palpebral muscle (Fig. 7). The thicknesses of the superior palpebral muscle were 0.14±0.13 mm at the anterior border of the superior transverse ligament, 0.45±0.11 mm at the superior fornix level, and 0.10±0.03 mm at the upper border of the tarsal plate [38].
Fig. 6.
Schema of a parasagittal section through the midpupillary line. The levator palpebrae superior muscle divides into superficial (S) and deep (D) parts beneath the superior transverse ligament. The levator aponeurosis originates from this superficial portion. The superior palpebral muscle originates from the deep portion. A, levator aponeurosis; M, superior palpebral involuntary muscle (Müller's muscle); LP, levator palpebrae muscle; SR, superior rectus muscle.
Fig. 7.
Schema of the superior palpebral muscle (M) and levator aponeurosis. (A) The width of the superior palpebral muscle was 15.6 mm at the anterior border of the superior transverse ligament and 22.3 mm at the upper border of the tarsal plate. The height was 13.7 mm. (B) The width of the levator aponeurosis was wider than that of the superior palpebral muscle. The width of the levator aponeurosis was 19.3 mm at the anterior border of the superior transverse ligament and 26.8 mm at the upper border of the tarsal plate. Medially, the levator aponeurosis was 1.9 and 1.6 mm wider at the level of the superior transverse ligament and the upper tarsal border, respectively. Laterally, the levator aponeurosis was 1.8 and 1.6 mm wider at the level of the superior transverse ligament and at the upper tarsal border, respectively. LP, levator palpebrae muscle.
Collin et al. [39] found that the Müller muscle was 0.1-0.5 mm in thickness. There are 2 layers of vascular connective tissue between Müller's muscle and the levator aponeurosis on one side and the conjunctival epithelium on the other [39]. Hwang et al. [38] also reported the existence of 2 vascular layers: one between the levator aponeurosis and the superior palpebral muscle (upper vascular layer) and the other between the superior palpebral muscle and the conjunctiva (lower vascular layer). At the level of the superior fornix, the upper and lower vascular layer thicknesses were 0.28±0.06 and 0.38±0.21 mm, respectively [38].
Conclusion
Here we have presented a review of the upper eyelid anatomy crucial to those involved with upper blepharoplasty or blepharoptosis surgery. We hope that this knowledge will aid surgeons in achieving successful outcomes.
Acknowledgements
The authors declare that they have no conflicts of interest to disclose. This study was supported by a grant from the National Research Foundation of Korea (KRF-2008-521-E00366).
References
- 1.Barker DE. Skin thickness in the human. Plast Reconstr Surg (1946) 1951;7:115–116. doi: 10.1097/00006534-195102000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Lee Y, Hwang K. Skin thickness of Korean adults. Surg Radiol Anat. 2002;24:183–189. doi: 10.1007/s00276-002-0034-5. [DOI] [PubMed] [Google Scholar]
- 3.Hwang K, Kim DJ, Hwang SH. Thickness of Korean upper eyelid skin at different levels. J Craniofac Surg. 2006;17:54–56. doi: 10.1097/01.scs.0000188347.06365.a0. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez-Ulloa M, Flores ES. Senility of the face: basic study to understand its causes and effects. Plast Reconstr Surg. 1965;36:239–246. doi: 10.1097/00006534-196508000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea. 1992;11:334–342. doi: 10.1097/00003226-199207000-00012. [DOI] [PubMed] [Google Scholar]
- 6.Hwang K, Kim DJ, Kim SK. Does the upper eyelid skin become thinner with age? J Craniofac Surg. 2006;17:474–476. doi: 10.1097/00001665-200605000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Lipham WJ, Tawfik HA, Dutton JJ. A histologic analysis and three-dimensional reconstruction of the muscle of Riolan. Ophthal Plast Reconstr Surg. 2002;18:93–98. doi: 10.1097/00002341-200203000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Sullivan L. Form follows function [Internet] Wikipedia; [cited 2010 Dec 15]. Available from: http://en.wikipedia.org/wiki/Form_follows_function. [Google Scholar]
- 9.McLoon LK, Wirtschafter JD. Regional differences in the orbicularis oculi muscle: conservation between species. J Neurol Sci. 1991;104:197–202. doi: 10.1016/0022-510x(91)90310-4. [DOI] [PubMed] [Google Scholar]
- 10.Goodmurphy CW, Ovalle WK. Morphological study of two human facial muscles: orbicularis oculi and corrugator supercilii. Clin Anat. 1999;12:1–11. doi: 10.1002/(SICI)1098-2353(1999)12:1<1::AID-CA1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 11.Hwang K, Huan F, Kim DJ. Muscle fiber types of human orbicularis oculi muscle. J Craniofac Surg. 2011;22:1827–1830. doi: 10.1097/SCS.0b013e31822e8468. [DOI] [PubMed] [Google Scholar]
- 12.Hwang K, Kim DJ, Hwang SH. Immunohistochemical study of differences between the muscle fiber types in the pars peripheralis and marginalis. J Craniofac Surg. 2007;18:591–593. doi: 10.1097/scs.0b013e318052ff59. [DOI] [PubMed] [Google Scholar]
- 13.Standring S. Gray's anatomy: the anatomical basis of clinical practice. 39th ed. Edinburgh: Elsevier; 2005. pp. 500–501.pp. 691 [Google Scholar]
- 14.Ramirez OM, Peña G. Frontalis muscle advancement: a dynamic structure for the treatment of severe congenital eyelid ptosis. Plast Reconstr Surg. 2004;113:1841–1849. doi: 10.1097/01.prs.0000117664.07831.48. [DOI] [PubMed] [Google Scholar]
- 15.Knize DM. An anatomically based study of the mechanism of eyebrow ptosis. Plast Reconstr Surg. 1996;97:1321–1333. doi: 10.1097/00006534-199606000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Karacalar A, Korkmaz A, Kale A, Kopuz C. Compensatory brow asymmetry: anatomic study and clinical experience. Aesthetic Plast Surg. 2005;29:119–123. doi: 10.1007/s00266-004-0086-5. [DOI] [PubMed] [Google Scholar]
- 17.Hwang K, Kim DJ, Hwang SH. Insertion of frontalis muscle relating to blepharoptosis repair. J Craniofac Surg. 2005;16:965–967. doi: 10.1097/01.scs.0000198623.69372.22. [DOI] [PubMed] [Google Scholar]
- 18.Rainin EA, Carlson BM. Postoperative diplopia and ptosis. A clinical hypothesis based on the myotoxicity of local anesthetics. Arch Ophthalmol. 1985;103:1337–1339. doi: 10.1001/archopht.1985.01050090089038. [DOI] [PubMed] [Google Scholar]
- 19.Kalichman MW. Physiologic mechanisms by which local anesthetics may cause injury to nerve and spinal cord. Reg Anesth. 1993;18(6 Suppl):448–452. [PubMed] [Google Scholar]
- 20.Hwang K, Lee DK, Chung IH, Lee SI. Patterns of oculomotor nerve distribution to the levator palpebrae superioris muscle, and correlation to temporary ptosis after blepharoplasty. Ann Plast Surg. 2001;47:381–384. doi: 10.1097/00000637-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Whitnall SE. A ligament acting as a check to the action of the levator palpebrae superioris muscle. J Anat Physiol. 1911;45(Pt 2):131–139. [PMC free article] [PubMed] [Google Scholar]
- 22.Hwang K, Kim DJ, Chung RS, Lee SI, Hiraga Y. An anatomical study of the junction of the orbital septum and the levator aponeurosis in Orientals. Br J Plast Surg. 1998;51:594–598. doi: 10.1054/bjps.1998.0300. [DOI] [PubMed] [Google Scholar]
- 23.Bron AJ, Tripathi RC, Tripathi BJ. Wolff's anatomy of the eye and orbit. 8th ed. London: Arnold; 1997. pp. 14pp. 145–146. [Google Scholar]
- 24.Werb A. Senile ptosis. Trans Ophthalmol Soc U K. 1985;104(Pt 1):22–25. [PubMed] [Google Scholar]
- 25.Hwang K, Kim DJ, Huan F, Han SH, Hwang SW. Width of the levator aponeurosis is broader than the tarsal plate. J Craniofac Surg. 2011;22:1061–1063. doi: 10.1097/SCS.0b013e3182112c0f. [DOI] [PubMed] [Google Scholar]
- 26.Lockwood CB. The anatomy of the muscles, ligaments, and fasclae of the orbit, including an account of the capsule of tenon, the check ligaments of the recti, and the suspensory ligaments of the eye. J Anat Physiol. 1885;20(Pt 1):i2–i25. [PMC free article] [PubMed] [Google Scholar]
- 27.Toldt C, Rosa AD, Paul E. An atlas human anatomy for students and physicians. 6th section. London: Rebman Limited; 1904. pp. 956u–956v. [Google Scholar]
- 28.Whitnall SE. The levator palpebrae superioris muscle: the attachments and relations of its aponeurosis. Ophthalmoscope. 1914;12:258–263. [Google Scholar]
- 29.Whitnall SE. The anatomy of the human orbit and accessory organs of vision. 2nd ed. London: Humphrey Milford; 1932. pp. 141–146. [Google Scholar]
- 30.Fink WH. An anatomic study of the check mechanism of the vertical muscles of the eyes. Am J Ophthalmol. 1957;44:800–809. doi: 10.1016/0002-9394(76)90785-6. [DOI] [PubMed] [Google Scholar]
- 31.Manson PN, Clifford CM, Su CT, Iliff NT, Morgan R. Mechanisms of global support and posttraumatic enophthalmos: I. The anatomy of the ligament sling and its relation to intramuscular cone orbital fat. Plast Reconstr Surg. 1986;77:193–202. [PubMed] [Google Scholar]
- 32.Ettl A, Priglinger S, Kramer J, Koornneef L. Functional anatomy of the levator palpebrae superioris muscle and its connective tissue system. Br J Ophthalmol. 1996;80:702–707. doi: 10.1136/bjo.80.8.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holmstrom H, Santanelli F. Suspension of the eyelid to the check ligament of the superior fornix for congenital blepharoptosis. Scand J Plast Reconstr Surg Hand Surg. 2002;36:149–156. doi: 10.1080/028443102753718023. [DOI] [PubMed] [Google Scholar]
- 34.Holmström H, Bernström-Lundberg C, Oldfors A. Anatomical study of the structures at the roof of the orbit with special reference to the check ligament of the superior fornix. Scand J Plast Reconstr Surg Hand Surg. 2002;36:157–159. doi: 10.1080/028443102753718032. [DOI] [PubMed] [Google Scholar]
- 35.Hwang K, Shin YH, Kim DJ. Conjoint fascial sheath of the levator and superior rectus attached to the conjunctival fornix. J Craniofac Surg. 2008;19:241–245. doi: 10.1097/scs.0b013e3181577b2e. [DOI] [PubMed] [Google Scholar]
- 36.Duke-Elder S, Wybar KC. System of ophthalmology. Vol. 2. St Louis: CV Mosby; 1961. The anatomy of the visual system; pp. 446–447. [Google Scholar]
- 37.Isaksson I. Studies on congenital genuine blepharoptosis. Morphological and functional investigations of the upper eyelid. Acta Ophthalmol Suppl. 1962;72:1–121. [PubMed] [Google Scholar]
- 38.Hwang K, Huan F, Kim DJ, Hwang SH. Size of the superior palpebral involuntary muscle (Müller muscle) J Craniofac Surg. 2010;21:1626–1629. doi: 10.1097/SCS.0b013e3181ec6b18. [DOI] [PubMed] [Google Scholar]
- 39.Collin JR, Beard C, Wood I. Experimental and clinical data on the insertion of the levator palpebrae superioris muscle. Am J Ophthalmol. 1978;85:792–801. doi: 10.1016/s0002-9394(14)78107-3. [DOI] [PubMed] [Google Scholar]