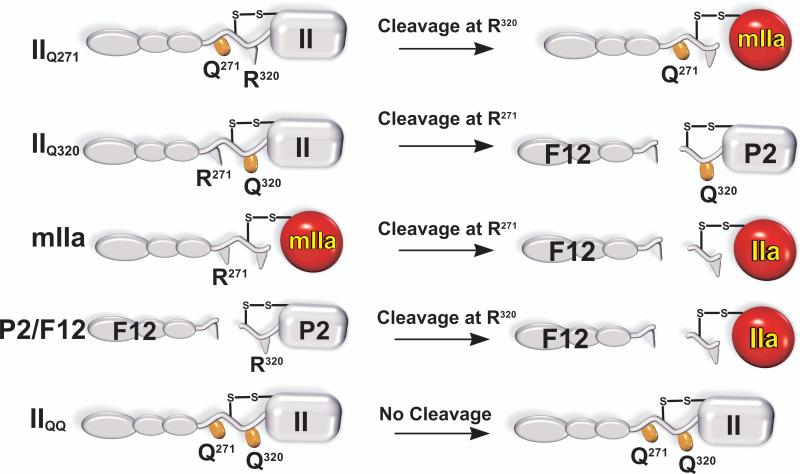
Figure 4. Substrate Derivatives for Kinetic Studies of all Possible Half-Reactions of Prothrombin Activation.
Cleavage of the individual sites in intact prothrombin was assessed using prothrombin variants in which the two arginines were individually rendered uncleavable by mutation to Gln. The intermediates mIIa and P2/F12 were used to assess cleavage at the individual sites following cleavage at the first site. IIQQ denotes a prothrombin variant in which both Arg side chains were mutated to Gln to yield an uncleavable derivative. The products formed upon the limiting action of prothrombinase on these substrate variants are illustrated.