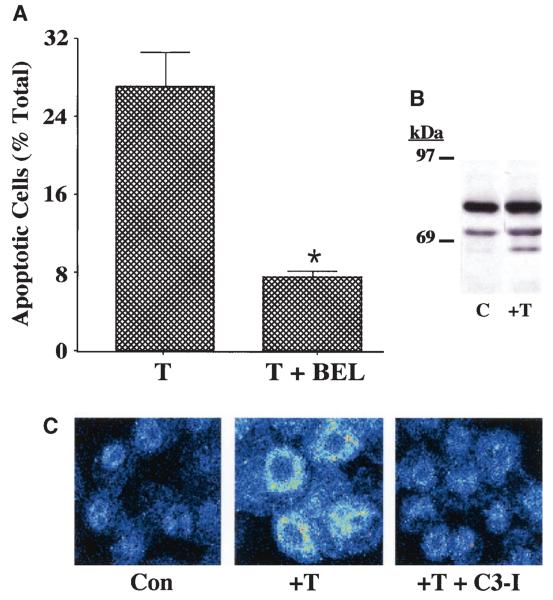
FIG. 3.
ER stress–induced apoptosis of INS-1 cells. A: Suppression of thapsigargin-induced apoptosis of iPLA2β-overexpressing INS-1 cells by BEL. INS-1 cells stably transfected with a vector containing iPLA2β cDNA (OE) construct were treated with thapsigargin (T) (1 μmol/l) for 24 h in the absence or presence of BEL (10 μmol/l). The cells were then harvested for TUNEL (Tdt-mediated dUTP nick end labeling) analyses to assess the magnitude of cell apoptosis (*T + BEL–treated group significantly different from T alone–treated group, P < 0.05). B: Immunoblotting analyses. Aliquots of INS-1 cell protein (50 μg), prepared from OE cells treated with dimethylsulfoxide (lane 1) or thapsigargin (1 μmol/l) (lane 2), were analyzed by SDS-PAGE (7.5%) and transferred onto an Immobolin-P polyvinylidene difluoride membrane. The electroblot was probed with piPLA2β antibodies, and immunoreactive protein bands were visualized by enhanced chemiluminescence, as described (41). C, control. C: Effects of caspase-3 inhibition on iPLA2β subcellular distribution. iPLA2β OE INS-1 cells seeded in glass chambered slides were pretreated with caspase-3 inhibitor (C3-I) (500 nmol/l) or vehicle for 24 h. The cells were then treated with thapsigargin in the absence and presence of C3-I for 24 h and subsequently processed for iPLA2β immunofluorescence analyses by confocal microscopy. Con, control.