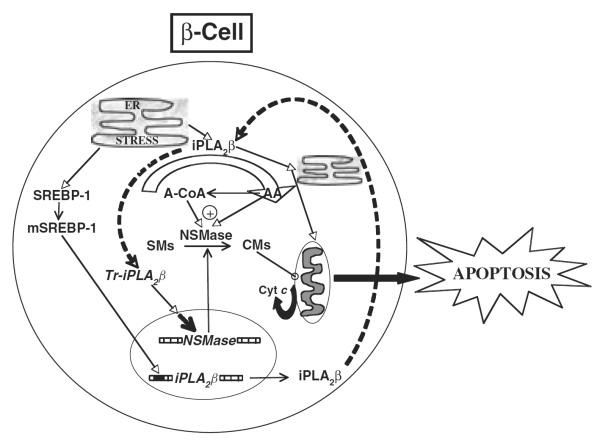
Figure 1.
Proposed role of the cytosolic group VIA Ca2+-independent phospholipase A2 (iPLA2β)–ceramide axis in endoplasmic reticulum (ER) stress-induced β-cell apoptosis. ER stress in the β-cell promotes the expression/activity of iPLA2β, leading to the induction of NSMase and increased hydrolysis of sphingomyelins from β-subcellular organelles and accumulation of ceramides in the cytosol. These lipid second messengers activate the mitochondrial apoptotic processes and cause loss in ΔΨ, activation of permeability transition pore and release of cytochrome c into the cytosol. The latter interacts with other factors to activate caspases leading to apoptosis of the β-cell. Consistent with a role for iPLA2β-mediated ceramide generation via NSMase in this process, inactivation of iPLA2β or chemical inhibition or knockdown of NSMase suppresses NSMase expression and ceramide generation, cytochrome c release and apoptosis [54]. The broken arrows linking iPLA2β and truncated iPLA2β (Tr-iPLA2β) indicate a series of events leading to the generation of a caspase-3-processed highly active iPLA2β.