Abstract
Abnormal responses of the brain to delivered and expected aversive gut stimuli have been implicated in the pathophysiology of irritable bowel syndrome (IBS), a visceral pain syndrome occurring more commonly in women. Task-free resting-state functional magnetic resonance imaging (fMRI) can provide information about the dynamics of brain activity that may be involved in altered processing and/or modulation of visceral afferent signals. Fractional amplitude of low-frequency fluctuation is a measure of the power spectrum intensity of spontaneous brain oscillations. This approach was used here to identify differences in the resting-state activity of the human brain in IBS subjects compared with healthy controls (HCs) and to identify the role of sex-related differences. We found that both the female HCs and female IBS subjects had a frequency power distribution skewed toward high frequency to a greater extent in the amygdala and hippocampus compared with male subjects. In addition, female IBS subjects had a frequency power distribution skewed toward high frequency in the insula and toward low frequency in the sensorimotor cortex to a greater extent than male IBS subjects. Correlations were observed between resting-state blood oxygen level-dependent signal dynamics and some clinical symptom measures (e.g., abdominal discomfort). These findings provide the first insight into sex-related differences in IBS subjects compared with HCs using resting-state fMRI.
Introduction
Irritable bowel syndrome (IBS) is the most common chronic visceral pain disorder and occurs with a greater prevalence in women (Mykletun et al., 2010; Chang, 2011; Fukudo and Kanazawa, 2011). Functional brain imaging studies have demonstrated greater blood oxygen level-dependent (BOLD) responses in IBS subjects in regions of a homeostatic afferent, emotional arousal, and cognitive modulatory networks (Wilder-Smith et al., 2004; Mayer and Bushnell, 2009; Tillisch et al., 2011). Sex-related differences in visceral perception, in the autonomic nervous system, and in brain responses to visceral stimuli or their expectation have been demonstrated (Mayer et al., 2001; Chang and Heitkemper, 2002; Mayer et al., 2004; Mayer et al., 2005; Mayer et al., 2006; Lieberman et al., 2007; Wang et al., 2007; Labus et al., 2008; van Marle et al., 2009). Similar to brain imaging studies in rodents, these studies have shown greater engagement of cortical regions in males (prefrontal cortical subregions) and greater engagement of emotional brain regions and circuits in females (amygdala [AMYG], subgenual cingulate cortex) during rectal distension and expectation of abdominal pain (Lawal et al., 2006; Naliboff et al., 2006).
Characterization of spontaneous intrinsic BOLD oscillations in the brain (resting-state functional magnetic resonance imaging [fMRI]) using different analytic approaches, including fractional amplitude of low-frequency fluctuation (fALFF), a measure of the power spectrum intensity of spontaneous brain frequency oscillations, have been used to identify brain abnormalities in psychiatric patients and in somatic pain conditions (Zang et al., 2007; Hoptman et al., 2010; Malinen et al., 2010; Davis and Moayedi, 2012; Farmer et al., 2012; Kwak et al., 2012; Wang et al., 2012). For example, patients with chronic spinal and limb pain have been shown to exhibit greater high-frequency (HF; 0.12–0.25 Hz) spectral power in the insula (INS) and anterior cingulate cortex (ACC) compared with healthy controls (HCs) (Malinen et al., 2010). Regional abnormalities in spontaneous BOLD oscillations in the INS, medial prefrontal cortex, and posterior cingulate cortex have also been observed in chronic back pain patients (Baliki et al., 2011). These alterations in regional frequency spectral power have been suggested to reflect abnormal intrinsic neuronal activities, functional connectivity, and spontaneous pain (Zou et al., 2008; Baliki et al., 2011).
In the present study, using fALFF, we aimed to identify disease-related regional differences in intrinsic oscillatory dynamics of BOLD signal as previously reported for other persistent pain conditions by comparing patients with IBS and HCs. In addition, we aimed to identify possible sex-related differences and to determine whether the identified regions with altered oscillatory dynamics are associated with IBS symptoms and behavioral characteristics. Based on previous published studies in chronic pain patients (Malinen et al., 2010; Baliki et al., 2011; Farmer et al., 2012), we aimed to test the following hypotheses using a region of interest (ROI) approach: (1) regarding disease-related differences, there is a shift of regional frequency spectral power toward HF in the interoceptive related regions in the patient group; (2) sex-related differences in oscillatory dynamics exist in emotional, somatosensory, and interoceptive regions; and (3) abnormal regional oscillatory dynamics are correlated with clinical symptoms.
Materials and Methods
Subjects.
A total of 178 right-handed subjects were recruited through the University of California-Los Angeles (UCLA) Digestive Diseases Clinic and advertisements. The sample included 76 female HCs (mean age, 29.39 ± 9.93 years), 42 male HCs (mean age, 35.95 ± 12.97 years), 29 male IBS subjects (mean age, 37.28 ± 10.75 years), and 31 female IBS subjects (mean age, 30.65 ± 10.71 years). Diagnosis of IBS was made by a gastroenterologist or nurse practitioner with expertise in functional gastrointestinal disorders based on the ROME II or ROME III symptom criteria during a clinical assessment (Drossman, 2000; Drossman, 2006). The diagnostic criteria include recurrent abdominal pain or discomfort associated with two or more of the following: (1) pain/discomfort is relieved/improved by defecation, (2) the onset of pain/discomfort is related to a change in frequency of stool, and (3) the onset of pain/discomfort is related to a change in the form (appearance) of stool. All procedures were approved by the UCLA Medical Institutional Review Board and all subjects provided informed consent. Exclusion criteria comprised pregnancy, substance abuse, abdominal surgery, tobacco dependence, and psychiatric illness. In addition, IBS subjects with current regular use of analgesic drugs (including narcotics, opioids, and α2-δ ligands) were excluded. Use of medications such as antidepressants (low-dose tricyclic antidepressants, selective serotonin uptake inhibitors, nonselective serotonin reuptake inhibitors) was only allowed if patients had been on a stable dose for a minimum of 3 months. In our sample, only three IBS subjects were on low-dose tricyclic antidepressants (one female on <75 mg/day) or selective serotonin uptake inhibitors (one female and one male). Questionnaires were completed before scanning to determine IBS symptom type, severity, duration of symptoms, and abdominal sensation using the UCLA Bowel Symptom Questionnaire (BSQ; Chang et al., 2001), comorbid affective and mood disorders using the Hospital Anxiety Depression (HAD) scale (Mykletun et al., 2001), IBS-related fears and anxiety using the Visceral Sensitivity Index (VSI; Labus et al., 2004; Labus et al., 2007), and the big five personality traits using the NEO Personality Inventory-Revised (NEO PI-R; Costa and McCrae, 1995).
Resting-state MRI data acquisition.
All resting-state scans were collected with subjects having their eyes closed while in the scanner. Noise-cancelling headphones were used to help reduce the noise from the scanner. Images were acquired with echo planar sequence on Siemens 3 Tesla Trio and Allegra scanners as follows: (1) Siemens 3 Tesla Trio using the following parameters: TE = 28 ms, TR = 2000 ms, scan duration = 10 m 6 s, flip angle = 77 degrees, FOV = 220, slice thickness = 4.0 mm, 40 slices were obtained with whole-brain coverage (27 female HCs, 28 male HCs, 19 male IBS subjects, 25 female IBS subjects); (2) Siemens 3 Tesla Trio using the following parameters: TE = 26 ms, TR = 2500 ms, scan duration = 5 m 8 s, flip angle = 90 degrees, FOV = 200, slice thickness = 3.0 mm, 38 slices were obtained with whole-brain coverage (41 female HCs); (3) Siemens 3 Tesla Trio using the following parameters: TE = 28 ms, TR = 2000 ms, scan duration = 8 m 6 s, flip angle = 77 degrees, FOV = 220, slice thickness = 4.0 mm, 40 slices were obtained with whole-brain coverage (8 female HCs, 6 female IBS subjects); and (4) Siemens 3 Tesla Allegra using the following parameters: TE = 28 ms, TR = 3000 ms, scan duration = 6 m 6 s, flip angle = 90 degrees, FOV = 200, slice thickness = 3.0 mm, 38 slices were obtained with whole-brain coverage. (14 male HCs, 10 male IBS subjects).
Structural MRI.
A standard T1-weighted magnetization-prepared rapid acquisition gradient echo (MP-RAGE) scan was obtained before resting scan session using the following parameters: TR = 2200 ms, TE = 3.26 ms, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 1.0 × 1.0 × 1.0 mm voxel size.
Resting-state MRI image preprocessing.
All image processing and data analysis were performed using Statistical Parametric Mapping 8 (SPM8) software (Wellcome Department of Cognitive Neurology, London). Images were first imported from DICOM into NIFTI-1 format followed by slice timing correction, spatial realignment, and motion correction. A native-to-MNI transformation matrix was obtained by running the MP-RAGE scan through a segmentation procedure. This matrix was then used to bring fMRI images, which were aligned to the MP-RAGE scan into MNI space. All scans were resampled to a voxel size of 2 × 2 × 2 mm.
Between group analyses of normalized fALFF.
We used fALFF, a power-density frequency spectrum approach applied to resting-state time course data, to identify region-specific abnormalities in resting-state brain function (Zou et al., 2008). The examination of low-frequency (LF) fluctuations of BOLD signal using this type of approach has exhibited a close relationship to spontaneous neuronal activity, adequate gray matter–white matter differentiation, and the improved fALFF algorithm specifically has been shown to reduce the confounding effects of physiological noise in the data compared with earlier frequency-based methods (Goldman et al., 2002; Mantini et al., 2007; Zou et al., 2008; Biswal et al., 2010). fALFF analysis was performed using in-house codes written in C++. Linear trends were removed before the time course data from each voxel was subjected to fast Fourier transformations into the frequency domain (Zou et al., 2008). Similar to many previous studies (Wise et al., 2004; Duff et al., 2008; Zou et al., 2008; Baliki et al., 2011; Baria et al., 2011), we subdivided the BOLD frequency into three different bands. We pooled resting-state fMRI data with different TRs (2–3 s) from multiple studies for the purposes of comparing patterns of BOLD oscillation in a large sample size. To make comparisons possible across multiple studies, we modified the fALFF by setting a cutoff frequency (0.16 Hz). The modified fALFF was calculated as the ratio of the square root of the oscillatory amplitude sum across the LF band (0.01–0.05 Hz), the middle frequency (MF) band (0.05–0.10 Hz), and the HF band (0.10–0.16 Hz) to the square root of the power across the entire frequency band (0–0.16 Hz). We normalized the fALFF for each voxel, transformed it to a Z-score by subtracting the global mean fALFF value, and then dividing by the SD within a whole brain mask (provided in MRIcro, http://www.mccauslandcenter.sc.edu/mricro/mricron/). An 8 mm isotropic Gaussian kernel was then used to smooth normalized fALFF maps (Zou et al., 2008).
Between-group analyses of normalized fALFF.
Group differences in fALFF in the different frequency bands were tested using linear contrast analyses on estimates from a general linear model implemented in SPM8. Using the flexible factorial specification, normalized fALFF maps were entered as dependent variables and group (male HC, female HC, male IBS, and female IBS) and frequency band (LF, MF, and HF) were entered as factors. The main effect and interactions between group and frequency band were entered as effects in the model. Subject was also included as an effect in the model (Gläscher and Gitelman, 2008). Because chronic pain has been associated with shifts in BOLD frequency power distribution (Malinen et al., 2010; Farmer et al., 2012), we specified interaction contrasts (i.e., differences of differences) to localize regions that exhibited changes/shifts in frequency power distribution due to group; for example: female HCs (HF vs LF) − female IBS (HF vs LF). As a sensitivity analysis, we entered subject age (in years), depression score, and factors for scanning protocols as nuisance covariates, but this did not change the results; for example, contrast maps for female IBS (HF vs LF) − male IBS (HF vs LF) showed 95.99% overlap. Therefore, we do not report results for the model controlling for these potential nuisance covariates.
Based on the findings from previous studies (Labus et al., 2008; Malinen et al., 2010; Apkarian et al., 2011; Baliki et al., 2011; Tillisch et al., 2011; Van Oudenhove, 2011), we investigated the resting-state signal in specific ROIs, including the sensorimotor cortex, subregions of INS (including anterior INS [aINS]; mid INS [mINS]; posterior INS [pINS]), and affective regions (AMYG and HIPP). ROIs were constructed in the WFU PickAtlas tool using the aal human brain atlas with a two-dimensional dilation factor of one (Maldjian et al., 2003; Maldjian et al., 2004). ROI analysis was applied by thresholding whole-brain fALFF activation maps at an uncorrected significance threshold of p < 0.001, using the small volume correction procedure in SPM8, and considering results as significant at a cluster threshold of p < 0.05 corrected for FWE correction. To better quantify the differences between groups, we extracted normalized fALFF values (Z-scores) averaged over the significant voxels within each ROI by MarsBaR (http://marsbar.sourceforge.net) and plotted them using SPSS version 19 software.
Independent sample t tests were conducted to examine bowel symptom severity (BSQ) between male and female IBS subjects. ANOVA was performed to examine differences in non-BSQ clinical and behavioral characteristics, including visceral sensitivity (VSI), anxiety and depression (HAD), and personality traits (NEO). Significant group effects were further examined using linear contrasts using false-discovery rate (FDR) for the four comparisons at 5% (Benjamini and Hochberg, 2000; Benjamini et al., 2006). Associations between clinical characteristics (BSQ, symptom duration, VSI, and HAD) and frequency oscillation shifts were conducted in SPSS by correlating clinical scores for each of the variables of interest with the frequency shifts between averaged Z-score of different fALFF maps for each voxel within the significant ROIs and correcting for multiple comparisons by FDR.
Results
Clinical characteristics
Subjects' clinical data are summarized in Table 1. Female IBS subjects tended to have greater IBS-related symptom scores compared with male IBS subjects (p > 0.05). Although within normal clinical ranges, IBS subjects had higher anxiety and depression symptom scores on the HAD than HCs, with male IBS patients having significantly higher scores than male HCs for anxiety (adjusted p (q) = 0.006), and female IBS having significantly higher anxiety (adjusted p (q) = 0.006) and depression (adjusted p (q) = 0.002) scores compared with female HCs. In addition, male and female IBS subjects had significantly higher VSI scores than male and female HCs, respectively (males: adjusted p (q) < 0.001; females: adjusted p (q) < 0.001).
Table 1.
Subject clinical and behavioral characteristics
| HC males |
IBS males |
HC females |
IBS females |
F | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |||
| Neuroticisma | 37 | 47.03 | 10.40 | 29 | 52.72 | 14.78 | 72 | 43.60 | 10.14 | 31 | 49.06 | 9.52 | 5.259 | 0.002 |
| Anxietyb | 42 | 3.26 | 2.60 | 29 | 5.69 | 4.45 | 76 | 3.04 | 2.436 | 31 | 5.03 | 3.67 | 7.123 | 0.000 |
| Depressionc | 42 | 1.64 | 1.56 | 29 | 2.79 | 3.40 | 76 | 1.09 | 1.73 | 31 | 2.42 | 2.50 | 5.475 | 0.001 |
| Overall Symptomsc | 24 | 9.71 | 4.24 | 31 | 9.97 | 4.38 | 0.058 | 0.826 | ||||||
| Abd Paind | 25 | 8.92 | 4.70 | 31 | 9.23 | 4.90 | 0.410 | 0.814 | ||||||
| Abd Discomforte | 25 | 8.80 | 5.66 | 31 | 11.58 | 5.15 | 0.668 | 0.060 | ||||||
| Durationf | 24 | 12.83 | 9.77 | 30 | 11.30 | 8.75 | 0.039 | 0.546 | ||||||
| Symptom related worriesg | 41 | 3.41 | 5.85 | 29 | 32.14 | 17.39 | 72 | 3.29 | 5.77 | 31 | 34.94 | 14.83 | 107.76 | 0.000 |
F is the main effect of group from ANOVA and t test for four and two group comparisons, respectively. Statistical significance was set at p < 0.05.
aNEO personality inventory (Costa and McCrae, 1995).
bHAD (Mykletun et al., 2001); BSQ (Chang et al., 2001).
cBSQ overall symptoms in the past week (0–20).
dBSQ abdominal pain in the past week (0–20).
eBSQ discomfort in the past week (0–20).
fBSQ duration in years, derived from onset of symptoms.
gVSI (Labus et al., 2004; Labus et al., 2007).
IBS-related differences in frequency power distribution
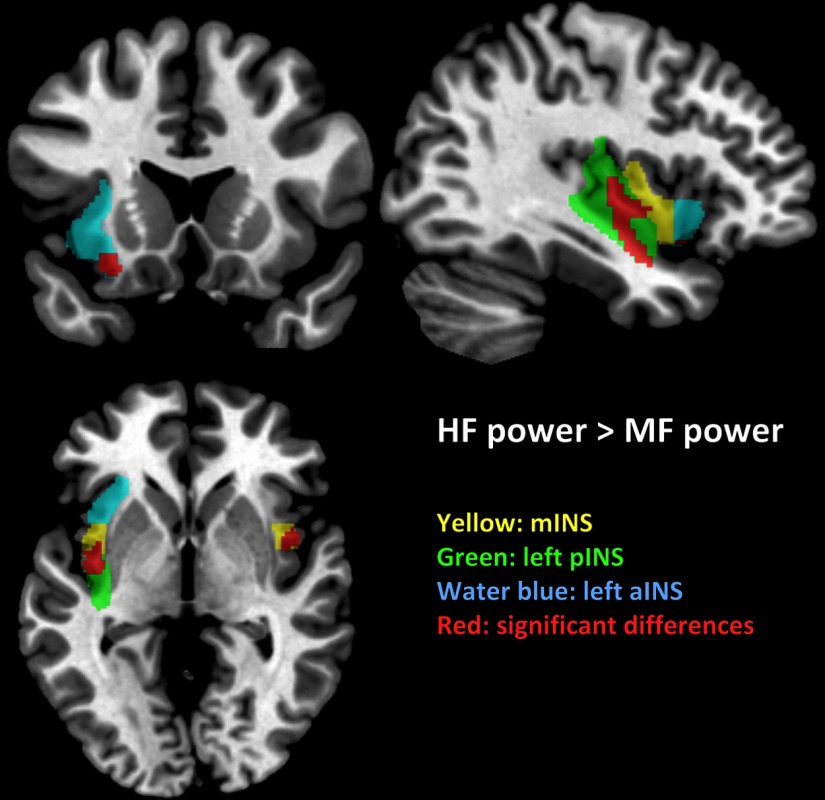
Male HCs showed greater HF versus MF and LF power distribution in the left aINS, bilateral mINS, and left pINS compared with male IBS subjects (Fig. 1 and Table 2). These results indicate that male HCs had a frequency power distribution skewed toward HF to a greater extent than male IBS subjects in the left aINS, bilateral mINS, and left pINS (Fig. 4a).
Figure 1.
Altered BOLD frequency power distribution of INS subregions in male subjects. Significant differences of HF versus MF power distribution in male HCs compared with male IBS subjects are shown in red (p < 0.05, FWE corrected). For display purposes only, all statistical results (p < 0.001 uncorrected) were overlapped on a MRIcron ch2better template.
Table 2.
Regions showing altered frequency power distribution in male IBS patients compared with male HCs
| ROI | Hemisphere | Cluster size (voxels) | p-value | T | Z-score | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Male HCs versus male IBS (amplitude of HF vs MF) | ||||||||
| aINS | L | 23 | 0.024 | 3.48 | 3.45 | −34 | 14 | −14 |
| mINS | L | 18 | 0.022 | 3.55 | 3.52 | −40 | −4 | 0 |
| pINS | L | 92 | 0.008 | 4.02 | 3.97 | −40 | −8 | 0 |
| mINS | R | 26 | 0.017 | 3.9 | 3.85 | 40 | −8 | 0 |
| Male HCs versus male IBS (amplitude of HF vs LF) | ||||||||
| pINS | L | 22 | 0.032 | 4.02 | 3.97 | −38 | −10 | 6 |
ROI analysis was applied by thresholding whole-brain fALFF activation maps at an uncorrected significance threshold of p < 0.001 and using the small volume correction procedure in SPM8. Results were considered significant at cluster threshold of p < 0.05 corrected for voxel wise error rate. MNI coordinates (x,y,z) for peak voxels show significance.
Figure 4.
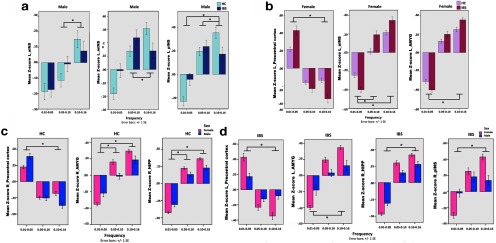
Comparison of frequency power in represented ROIs across groups. Graphs show normalized mean Z-score of frequency power distribution for selected ROIs. a, Male HCs showed greater frequency power distributions skewed from MF toward HF in left INS subregions. b, Female IBS showed greater frequency power distributions skewed toward HF in left AMYG and aINS, and toward LF in left precentral cortex compared with female HCs. c, Female HCs had greater frequency power shifts from LF toward higher frequencies in right AMYG and right HIPP compared with Male HCs. Male HCs had a greater skew from HF toward LF in right precentral cortex compared with female HCs. d, Female IBS subjects showed greater frequency power oscillation skewed toward HF in right pINS, right HIPP, and left AMYG and toward LF power in left precentral cortex compared with male IBS. *Significant difference, p < 0.05, FWE corrected.
Female IBS patients showed greater HF and MF versus LF power distribution in the left AMYG, right HIPP, and aINS compared with female HCs (Fig. 2a and Table 3). ROI analysis also revealed that female IBS subjects had greater frequency distribution of LF versus MF and HF power in the sensorimotor regions (precentral, postcentral, and paracentral cortex and supplementary motor area) compared with female HCs (Fig. 2b and Table 3). These results indicate that female IBS had a frequency power distribution skewed toward HF in the left AMYG, right HIPP, and aINS and toward LF in the sensorimotor regions to a greater extent compared with female HCs (Fig. 4b).
Figure 2.
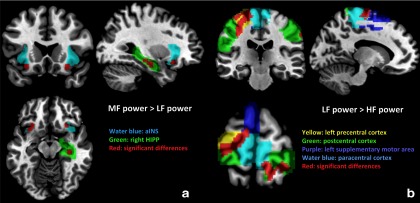
Altered BOLD frequency power distribution in female IBS compared with female HCs. a, Significant differences of MF versus LF power distribution in female IBS compared with female HCs are shown in red (p < 0.05, FWE corrected). b, ROIs with significant differences between LF versus HF power distribution in female IBS compared with female HCs are shown in red (p < 0.05, FWE corrected).
Table 3.
Regions showing altered frequency power distribution in female IBS patients compared with female HCs
| ROI | Hemisphere | Cluster size (voxels) | p-value | T | Z-score | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Female IBS versus female HCs (amplitude of MF vs LF) | ||||||||
| aINS | L | 51 | 0.013 | 4.64 | 4.57 | −28 | 20 | −18 |
| aINS | R | 33 | 0.019 | 4.1 | 4.05 | 28 | 22 | −16 |
| HIPP | R | 23 | 0.011 | 3.69 | 3.65 | 34 | −24 | −14 |
| Female IBS versus female HCs (amplitude of HF vs LF) | ||||||||
| aINS | L | 6 | 0.042 | 3.4 | 3.37 | −28 | 20 | −18 |
| AMYG | L | 6 | 0.016 | 3.27 | 3.24 | −26 | −4 | −24 |
| Female IBS versus female HCs (amplitude of LF vs MF) | ||||||||
| paCC | L | 28 | 0.041 | 3.63 | 3.60 | −6 | −40 | 62 |
| paCC | R | 18 | 0.041 | 3.58 | 3.30 | 10 | −42 | 68 |
| Female IBS versus female HCs (amplitude of LF vs HF) | ||||||||
| paCC | L | 113 | 0.008 | 4.14 | 4.09 | −16 | −14 | 78 |
| paCC | R | 81 | 0.011 | 4.26 | 4.20 | 10 | −44 | 70 |
| poCC | L | 166 | 0.010 | 4.15 | 4.10 | −34 | −30 | 70 |
| poCC | R | 197 | 0.007 | 4.26 | 4.20 | 10 | −44 | 70 |
| preCC | L | 444 | 0.000 | 4.18 | 4.12 | −18 | −14 | 78 |
| SMA | L | 35 | 0.049 | 3.95 | 3.91 | −14 | −12 | 76 |
ROI analysis was applied by thresholding whole-brain fALFF activation maps at an uncorrected significance threshold of p < 0.001 and using the small volume correction procedure in SPM8. Results were considered significant at cluster threshold of p < 0.05 corrected for voxel wise error rate. MNI coordinates (x,y,z) for peak voxels show significance. paCC indicates paracentral cortex; preCC, precentral cortex; poCC, postcentral cortex; SMA, supplementary motor area.
There were no significant differences between IBS subjects and HCs when males and females were combined in each group.
Sex-related differences in frequency power distribution
HCs
Male subjects showed greater LF versus HF power distribution in sensorimotor cortical regions (bilateral precentral and right postcentral cortex) compared with female subjects (Table 4). Female subjects had larger HF and MF versus LF power distribution in AMYG and HIPP, compared with males (Table 4). These results indicate that male HCs had a frequency power distribution skewed toward LF in the sensorimotor cortex to a greater extent than female HCs, whereas female HCs had a distribution skewed toward HF in affective regions to a greater extent than in male HCs (Fig. 4c).
Table 4.
Brain regions showing sex differences in frequency power distribution in HCs
| ROI | Hemisphere | Cluster size (voxels) | p-value | T | Z-score | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Female HCs versus male HCs (amplitude of MF vs LF) | ||||||||
| AMYG | L | 34 | 0.007 | 4.23 | 4.17 | −22 | −10 | −12 |
| HIPP | L | 28 | 0.01 | 4.50 | 4.44 | −26 | −18 | −16 |
| AMYG | R | 50 | 0.005 | 4.91 | 4.83 | 24 | −10 | −12 |
| HIPP | R | 52 | 0.006 | 5.3 | 5.19 | 28 | −16 | −14 |
| Female HCs versus male HCs (amplitude of HF vs LF) | ||||||||
| AMYG | L | 13 | 0.012 | 3.55 | 3.51 | −20 | −10 | −12 |
| HIPP | L | 15 | 0.015 | 3.78 | 3.74 | −26 | −18 | −16 |
| HIPP | R | 67 | 0.004 | 4.64 | 4.57 | 28 | −16 | −20 |
| Male HCs versus female HCs (amplitude of LF vs HF) | ||||||||
| poCC | R | 149 | 0.013 | 4.42 | 4.35 | 66 | −20 | 40 |
| preCC | L | 109 | 0.021 | 4.39 | 4.33 | −48 | 4 | 54 |
| preCC | R | 131 | 0.015 | 5.39 | 5.28 | 50 | 14 | 38 |
ROI analysis was applied by thresholding whole-brain fALFF activation maps at an uncorrected significance threshold of p < 0.001 and using the small volume correction procedure in SPM8. Results were considered significant at cluster threshold of p < 0.05 corrected for voxel wise error rate. MNI coordinates (x,y,z) for peak voxels show significance.
IBS group
Female compared with male IBS subjects had greater HF versus LF power distribution in all INS subregions (aINS, mINS, and pINS) and several affective regions (AMYG and HIPP) (Fig. 3a and Table 5). In addition, female subjects showed significantly greater LF versus HF power distribution in the left precentral, postcentral cortex, and supplementary motor area (Fig. 3b and Table 5). These results indicate that female IBS subjects had a frequency power distribution skewed toward LF in sensorimotor regions, as well as toward HF in the interoceptive and emotional arousal regions to a greater extent than in male IBS subjects (Fig. 4d).
Figure 3.
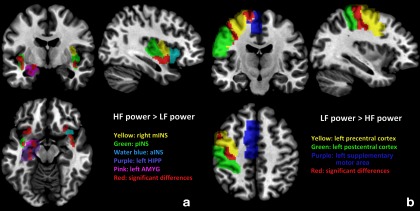
Altered regional BOLD oscillation distribution in female IBS subjects compared with male IBS subjects. a, Significant differences of HF versus LF power distribution in female IBS subjects compared with male IBS subjects are shown in red (p < 0.05, FWE corrected). b, ROIs with significant differences between LF versus HF power distribution in female IBS subjects compared with male IBS subjects are shown in red (p < 0.05, FWE corrected).
Table 5.
Brain regions showing sex differences in frequency power distribution in IBS
| ROI | Hemisphere | Cluster size (voxels) | p-value | T | Z-score | x | y | z |
|---|---|---|---|---|---|---|---|---|
| Female IBS versus male IBS (amplitude of HF vs LF) | ||||||||
| aINS | L | 52 | 0.013 | 3.89 | 3.84 | −32 | 20 | −16 |
| pINS | L | 53 | 0.016 | 3.47 | 3.44 | −36 | −6 | −12 |
| aINS | R | 44 | 0.015 | 4.54 | 4.48 | 30 | 22 | −16 |
| mINS | R | 82 | 0.005 | 4.00 | 3.95 | 36 | 6 | −12 |
| pINS | R | 115 | 0.005 | 4.36 | 4.30 | 38 | −4 | −14 |
| AMYG | L | 27 | 0.008 | 3.44 | 3.41 | −30 | −2 | −18 |
| HIPP | L | 46 | 0.007 | 4.02 | 3.98 | −26 | −26 | −10 |
| Female IBS versus male IBS (amplitude of LF vs HF) | ||||||||
| poCC | L | 287 | 0.002 | 4.56 | 4.50 | −34 | −16 | 40 |
| preCC | L | 544 | 0.000 | 4.93 | 4.84 | −18 | −20 | 68 |
| SMA | L | 67 | 0.026 | 4.51 | 4.44 | −14 | −14 | 72 |
ROI analysis was applied by thresholding whole-brain fALFF activation maps at an uncorrected significance threshold of p < 0.001 and using the small volume correction procedure in SPM8. Results were considered significant at cluster threshold of p < 0.05 corrected for voxel wise error rate. MNI coordinates (x,y,z) for peak voxels show significance.
Correlations of BOLD signal dynamics with clinical and behavioral characteristics
Correlation analyses were performed separately in male and female subjects between clinical symptom scores and the frequency power distributions from regions displaying sex-specific IBS-related alterations. In female IBS subjects, IBS symptom-related discomfort level during the past week was positively correlated with the degree to which the frequency power distribution was skewed toward HF (HF vs LF) in the left aINS (r = 0.506, p = 0.003, q = 0.04, FDR corrected). In male IBS subjects, IBS symptom-related discomfort level was associated with the degree of frequency power distribution (HF vs LF) in the left HIPP. However, this correlation did not survive FDR correction (r = 0.52, p = 0.008, q = 0.086). No statistically significant correlations between regional frequency power abnormalities in a priori brain regions (e.g., pINS in males or aINS in females) and any other clinical variables (including IBS symptom duration, NEO-Neuroticism, HAD anxiety, or symptom-related anxiety) were identified.
Discussion
In the present study, we examined group differences between patients with persistent abdominal pain (IBS) and HCs in the distribution of intrinsic BOLD oscillations across three frequency bands using a ROI approach, with a special emphasis on detecting sex-related differences in these patterns, resulting in the following main findings. First, significant disease-related differences were observed when female IBS subjects were compared with female HCs, with patients showing a greater skew in the frequency power distribution toward HF in AMYG and aINS and a greater skew toward LF in the sensorimotor regions. Second, significant sex-related differences were observed both within the HC and the IBS groups: female HCs (compared with males) showed a greater skew in the frequency power distribution toward HF in AMYG and HIPP. Within the IBS group, female IBS subjects (compared with males) showed similar patterns as the observed sex differences within the HCs in regions of an emotional arousal circuit, in addition to a greater skew toward HF in all INS subregions. Third, altered regional frequency power distribution was correlated with subjective abdominal discomfort ratings in female patients, but not symptom duration or anxiety ratings. To our knowledge, this is the first report on sex- and disease-related differences in spontaneous brain oscillations in a large sample of male and female subjects with and without IBS.
The finding that some regions were dominated by LF oscillations whereas others were skewed toward HF is consistent with previous reports in other chronic pain conditions (Baria et al., 2011). For example, Baria et al. (2011) reported that unimodal brain regions (such as the sensorimotor cortex) tend to be dominated by LF oscillations, whereas more complex multimodal regions (e.g., paralimbic and limbic cortices) displayed a shift of power to HF bands. In addition, our results indicate that sex and diagnosis can alter the degree to which frequency power distribution is skewed.
Sex-specific alterations in oscillatory dynamics between IBS and HCs
In contrast to previous reports from other chronic pain conditions, no disease-related differences in regional oscillation frequencies were identified when male and female subjects were combined, despite the significantly larger sample size compared with previous reports. Even though these earlier studies in patients with different persistent pain conditions have provided evidence for abnormal resting-state oscillatory dynamics (Malinen et al., 2010; Baliki et al., 2011), the majority of studies were limited by small sample size, sometimes heterogeneous patient populations, and by the combination of male and female subjects.
When female and male subjects were compared separately, robust differences between IBS subjects and HCs were observed. Female IBS subjects showed oscillatory alterations in affect related brain regions (aINS and AMYG) and sensorimotor regions compared with female HCs. In contrast, male HCs showed alterations in viscerosensory regions (mainly in mINS and pINS) compared with male IBS subjects. Similar to previous observations (Malinen et al., 2010; Napadow et al., 2010; Baliki et al., 2011; Ichesco et al., 2012), we observed disease-related alterations in oscillatory dynamics in the INS, even though the affected INS subregions differed between males and females. Although pINS and mINS receive primary interoceptive and somatosensory inputs, the aINS is considered a multimodal association cortex receiving interoceptive and affective inputs (Craig, 2009). One may speculate that an upregulation of emotional arousal/salience circuits involving the aINS and AMYG contributes more to symptoms in female patients, whereas an increased engagement of networks related to sensorimotor integration and interoceptive processing may play a greater role in male patients.
Sex-related differences in HCs
The present study found significant sex-related differences associated with spontaneous BOLD fluctuation patterns in the HC group. Female subjects showed different oscillation dynamics compared with males in affect-related regions and in sensorimotor regions. The pattern of the observed sex-related difference in intrinsic BOLD oscillations are similar to those reported from studies on brain responses to emotional stimuli (Kilpatrick et al., 2006; Dickie and Armony, 2008; Stevens and Hamann, 2012) and pain stimuli (Derbyshire et al., 2002; Moulton et al., 2006) in healthy subjects. For example, in response to negative emotional stimuli, female subjects had greater activation in HIPP and AMYG compared with male subjects (Stevens and Hamann, 2012). In response to noxious heat, male HCs had greater activations in somatosensory and viscerosensory regions, as well as the prefrontal and parietal regions compared with female HCs (Derbyshire et al., 2002; Moulton et al., 2006). The similarity between sex differences in resting brain measures and task-based measures is consistent with previous studies demonstrating a relationship between rest and task-evoked responses (Mennes et al., 2011), even though the precise relationship between task-based BOLD signals and resting-state frequency fluctuations remains to be determined (Mennes et al., 2011). In summary, our findings suggest a fundamental difference in the central processing of affective and nociceptive stimuli in the healthy male and female brain, which can be detected in the absence of any stimulus.
Sex-related differences in IBS subjects
Similar to the healthy group, female IBS subjects (compared with males) had greater oscillatory dynamics in affect-related regions (AMYG and HIPP) and in all INS subregions, which were not observed in HCs. The current findings suggest some similarities with previously published sex-related differences observed in evoked brain responses in IBS subjects (Naliboff et al., 2003; Labus et al., 2008). Abnormal power spectral density shifts from LF toward HF, especially at 0.16 Hz in the INS, had been reported previously in patients with chronic musculoskeletal pain (Malinen et al., 2010). Our study also showed that IBS subjects had similar aberrant frequency power distributions in all the INS subregions, with female IBS subjects showing greater fluctuation shifts from LF to HF. Even though the reason behind the greater frequency dynamics in female IBS subjects remains unclear, one can speculate that increased activity in affective regions (e.g., AMYG and HIPP) contributed to this sex-related difference in frequency power distributions of the anterior aspects of the INS.
Correlation of frequency dynamics with clinical/behavioral parameters
In female patients, a significantly positive correlation between aberrant frequency power distribution in the left aINS and the subjective report of abdominal discomfort was observed, consistent with previous reports on correlations of the aINS with subjective ratings of visceral stimuli (Dunckley et al., 2005; Lowen et al., 2013). In view of the close bidirectional interactions of aINS with the AMYG, it is conceivable that tonically increased input to the aINS from emotional arousal circuits plays a role both in the generation of subjective symptoms and in the observed abnormalities in intrinsic oscillations. The lack of a significant correlation between symptom duration and altered oscillatory dynamics makes it less likely that the observed findings are a consequence of longstanding alteration in gut function and visceral afferent signaling, and one could speculate that it represents a primary, possibly genetically/epigenetically determined abnormality of central sensory processing. Alternatively, the lack of significant correlations with clinical symptoms, including duration, anxiety, or NEO-Neuroticism, may be related to sample size or to the fact that such complex clinical variables are more correlated with alterations in brain networks than with activity in individual regions.
Possible pathophysiological implications
The findings of this study are consistent with a dysregulation of at least two resting-state networks involving affective and sensory brain regions. Evidence has been provided for the existence of two distinct and anticorrelated intrinsic resting-state networks associated with different INS subregions that function as hubs. One network is centered in the ventral aspect of the aINS with connections to the ACC, AMYG, and temporal lobe regions, is primarily related to attentional and affective regions, and may play a role in salience detection and other emotional aspects. The other network links the mINS and pINS to sensorimotor, premotor, MCC, and pACC, indicating a role in sensorimotor integration (Cauda et al., 2011). The fact that previous work has shown good correspondence of networks derived from resting-state studies and from task-relevant brain activation studies (De Luca et al., 2005; Vincent et al., 2006; Fox and Raichle, 2007; Smith et al., 2009; Baria et al., 2011) is consistent with our observation that disease- and sex-related findings in the current resting-state study are similar to previously published activation studies in IBS.
Conclusions
To our knowledge, this is the first large scale resting-state fMRI analysis performed in IBS subjects identifying disease-related oscillation frequencies that are sex specific. The findings are consistent with sex-specific dysregulations in INS centric networks engaged in emotional arousal/salience detection and in sensorimotor processing (Cauda et al., 2011; Cauda et al., 2012), confirming previous findings obtained in studies using evoked brain responses to nociceptive stimuli and their expectation. The lack of identified correlations between these frequency abnormalities and symptom duration, together with the observation that several of the observed sex-related differences in IBS were also observed in the HCs, suggests that some of these alterations may be primary and not a consequence of longstanding symptoms.
Footnotes
This work was supported by the National Institutes of Health (Grants #R01 DK048351, #P50 DK064539, #U01 DK082370, #P30 DK041301, #R03 DK084169, and #K01 DK085133). Pilot scanning was provided by the UCLA Ahmanson-Lovelace Brain Mapping Center.
The authors declare no competing financial interests.
References
- Apkarian AV, Hashmi JA, Baliki MN. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain. 2011;152:S49–S64. doi: 10.1016/j.pain.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Baria AT, Apkarian AV. The cortical rhythms of chronic back pain. J Neurosci. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baria AT, Baliki MN, Parrish T, Apkarian AV. Anatomical and functional assemblies of brain BOLD oscillations. J Neurosci. 2011;31:7910–7919. doi: 10.1523/JNEUROSCI.1296-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics. 2000;25:60–83. doi: 10.2307/1165312. [DOI] [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. doi: 10.1093/biomet/93.3.491. [DOI] [Google Scholar]
- Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li SJ, Lin CP, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D'Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Cauda F, Costa T, Torta DM, Sacco K, D'Agata F, Duca S, Geminiani G, Fox PT, Vercelli A. Meta-analytic clustering of the INS cortex: characterizing the meta-analytic connectivity of the insula when involved in active tasks. Neuroimage. 2012;62:343–355. doi: 10.1016/j.neuroimage.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Heitkemper MM. Gender differences in irritable bowel syndrome. Gastroenterology. 2002;123:1686–1701. doi: 10.1053/gast.2002.36603. [DOI] [PubMed] [Google Scholar]
- Chang L, Lee OY, Naliboff B, Schmulson M, Mayer EA. Sensation of bloating and visible abdominal distension in patients with irritable bowel syndrome. Am J Gastroenterol. 2001;96:3341–3347. doi: 10.1111/j.1572-0241.2001.05336.x. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Domains and facets: hierarchical personality assessment using the revised NEO personality inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Davis KD, Moayedi M. Central mechanisms of pain revealed through functional and structural MRI. J Neuroimmune Pharmacol. 2012;8:518–534. doi: 10.1007/s11481-012-9386-8. [DOI] [PubMed] [Google Scholar]
- De Luca M, Smith S, De Stefano N, Federico A, Matthews PM. Blood oxygenation level dependent contrast resting state networks are relevant to functional activity in the neocortical sensorimotor system. Exp Brain Res. 2005;167:587–594. doi: 10.1007/s00221-005-0059-1. [DOI] [PubMed] [Google Scholar]
- Derbyshire SW, Nichols TE, Firestone L, Townsend DW, Jones AK. Gender differences in patterns of cerebral activation during equal experience of painful laser stimulation. J Pain. 2002;3:401–411. doi: 10.1054/jpai.2002.126788. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Armony JL. Amygdala responses to unattended fearful faces: Interaction between sex and trait anxiety. Psychiatry Res. 2008;162:51–57. doi: 10.1016/j.pscychresns.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Drossman DA. Rome II: the functional gastrointestinal disorders: diagnosis, pathophysiology, and treatment: a multinational consensus. Ed 2. McLean, VA: Degnon Associates; 2000. [Google Scholar]
- Drossman DA. The functional gastrointestinal disorders and the Rome III process. Gastroenterology. 2006;130:1377–1390. doi: 10.1053/j.gastro.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Hum Brain Mapp. 2008;29:778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience. 2005;133:533–542. doi: 10.1016/j.neuroscience.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Farmer MA, Baliki MN, Apkarian AV. A dynamic network perspective of chronic pain. Neurosci Lett. 2012;520:197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroenterol Hepatol. 2011;26:110–115. doi: 10.1111/j.1440-1746.2011.06631.x. [DOI] [PubMed] [Google Scholar]
- Gläscher J, Gitelman D. Contrast weights in flexible factorial design with multiple groups of subjects. 2008. Retrieved May 10, 2010, from http://www.sbirc.ed.ac.uk/cyril/fMRI.html.
- Goldman RI, Stern JM, Engel J, Jr, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002;13:2487–2492. doi: 10.1097/00001756-200212200-00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophr Res. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichesco E, Quintero A, Clauw DJ, Peltier S, Sundgren PM, Gerstner GE, Schmidt-Wilcke T. Altered functional connectivity between the insula and the cingulate cortex in patients with temporomandibular disorder: a pilot study. Headache. 2012;52:441–454. doi: 10.1111/j.1526-4610.2011.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage. 2006;30:452–461. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Kwak Y, Peltier SJ, Bohnen NI, Müller ML, Dayalu P, Seidler RD. L-DOPA changes spontaneous low-frequency BOLD signal oscillations in Parkinson's disease: a resting state fMRI study. Front Syst Neurosci. 2012;6:52. doi: 10.3389/fnsys.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labus JS, Bolus R, Chang L, Wiklund I, Naesdal J, Mayer EA, Naliboff BD. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20:89–97. doi: 10.1111/j.1365-2036.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69:89–98. doi: 10.1097/PSY.0b013e31802e2f24. [DOI] [PubMed] [Google Scholar]
- Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal A, Kern M, Sidhu H, Hofmann C, Shaker R. Novel evidence for hypersensitivity of visceral sensory neural circuitry in irritable bowel syndrome patients. Gastroenterology. 2006;130:26–33. doi: 10.1053/j.gastro.2005.10.020. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Lowen MB, Mayer EA, Sjoberg M, Tillisch K, Naliboff B, Labus J, Lundberg P, Strom M, Engstrom M, Walter SA. Effect of hypnotherapy and educational intervention on brain response to visceral stimulus in the irritable bowel syndrome. Aliment Pharmacol Ther. 2013 doi: 10.1111/apt.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/S1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Burdette JH. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21:450–455. doi: 10.1016/j.neuroimage.2003.09.032. [DOI] [PubMed] [Google Scholar]
- Malinen S, Vartiainen N, Hlushchuk Y, Koskinen M, Ramkumar P, Forss N, Kalso E, Hari R. Aberrant temporal and spatial brain activity during rest in patients with chronic pain. Proc Natl Acad Sci U S A. 2010;107:6493–6497. doi: 10.1073/pnas.1001504107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A. 2007;104:13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Chang L, Coutinho SV. V. Stress and irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Chang L, Naliboff BD. Sex-based differences in gastrointestinal pain. Eur J Pain. 2004;8:451–463. doi: 10.1016/j.ejpain.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Berman S, Suyenobu B, Labus J, Mandelkern MA, Naliboff BD, Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC International Association for the Study of Pain. Functional pain syndromes: presentation and pathophysiology. Seattle: IASP; 2009. [Google Scholar]
- Mennes M, Zuo XN, Kelly C, Di Martino A, Zang YF, Biswal B, Castellanos FX, Milham MP. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage. 2011;54:2950–2959. doi: 10.1016/j.neuroimage.2010.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton EA, Keaser ML, Gullapalli RP, Maitra R, Greenspan JD. Sex differences in the cerebral BOLD signal response to painful heat stimuli. Am J Physiol Regul Integr Comp Physiol. 2006;291:R257–R267. doi: 10.1152/ajpregu.00084.2006. [DOI] [PubMed] [Google Scholar]
- Mykletun A, Stordal E, Dahl AA. Hospital Anxiety and Depression (HAD) scale: factor structure, item analyses and internal consistency in a large population. Br J Psychiatry. 2001;179:540–544. doi: 10.1192/bjp.179.6.540. [DOI] [PubMed] [Google Scholar]
- Mykletun A, Jacka F, Williams L, Pasco J, Henry M, Nicholson GC, Kotowicz MA, Berk M. Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS). An epidemiological population based study of women. BMC Gastroenterol. 2010;10:88. doi: 10.1186/1471-230X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Chang L, Derbyshire SW, Suyenobu B, Vogt BA, Mandelkern M, Mayer EA. Sex-related differences in IBS patients: central processing of visceral stimuli. Gastroenterology. 2003;124:1738–1747. doi: 10.1016/S0016-5085(03)00400-1. [DOI] [PubMed] [Google Scholar]
- Naliboff BD, Berman S, Suyenobu B, Labus JS, Chang L, Stains J, Mandelkern MA, Mayer EA. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131:352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marle HJ, Hermans EJ, Qin S, Fernández G. From specificity to sensitivity: how acute stress affects amygdala processing of biologically salient stimuli. Biolo Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Van Oudenhove L. Understanding gut-brain interactions in gastrointestinal pain by neuroimaging: lessons from somatic pain studies. Neurogastroenterol Motil. 2011;23:292–302. doi: 10.1111/j.1365-2982.2010.01666.x. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Social cognitive and affective neuroscience. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Dai W, Su Y, Wang G, Tan Y, Jin Z, Zeng Y, Yu X, Chen W, Wang X, Si T. Amplitude of low-frequency oscillations in first-episode, treatment-naive patients with major depressive disorder: a resting-state functional MRI study. PLoS One. 2012;7:e48658. doi: 10.1371/journal.pone.0048658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilder-Smith CH, Schindler D, Lovblad K, Redmond SM, Nirkko A. Brain functional magnetic resonance imaging of rectal pain and activation of endogenous inhibitory mechanisms in irritable bowel syndrome patient subgroups and healthy controls. Gut. 2004;53:1595–1601. doi: 10.1136/gut.2003.028514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RG, Ide K, Poulin MJ, Tracey I. Resting fluctuations in arterial carbon dioxide induce significant low frequency variations in BOLD signal. Neuroimage. 2004;21:1652–1664. doi: 10.1016/j.neuroimage.2003.11.025. [DOI] [PubMed] [Google Scholar]
- Zang YF, He Y, Zhu CZ, Cao QJ, Sui MQ, Liang M, Tian LX, Jiang TZ, Wang YF. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain Dev. 2007;29:83–91. doi: 10.1016/j.braindev.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Zou QH, Zhu CZ, Yang Y, Zuo XN, Long XY, Cao QJ, Wang YF, Zang YF. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. J Neurosci Methods. 2008;172:137–141. doi: 10.1016/j.jneumeth.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]