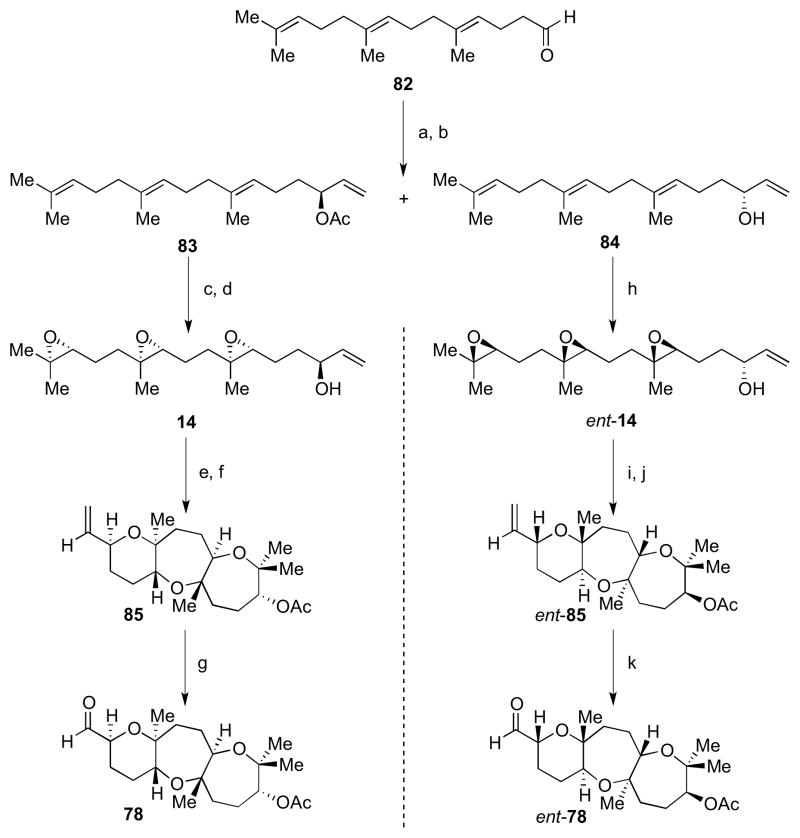
Scheme 20.
Syntheses of fused tricycles 78 and ent-78. Reagents and conditions: a) CH2CHMgBr, THF, −10 °C to rt, 73%; b) Novozyme 435, vinyl acetate, Et2O, 4 °C, 40%, 99% ee (for 83), 33%, 98% ee (for 84); c) Shi ketone (36), Oxone, nBu4NHSO4, aq. K2CO3, Na2B4O7 buffer, pH 10.5, (CH3O)2CH2/CH3CN/H2O, rt, 88%, 3.5:1 dr; d) LiOH, THF/MeOH/H2O, 0 °C, 93%; e) BF3•OEt2, CH2Cl2, −78 °C; f) Ac2O, Et3N, DMAP, CH2Cl2, rt, 18% from 14; g) (i) O3, NaHCO3, CH2Cl2/MeOH, −78 °C; (ii) PPh3, −78 °C to rt, 65%; h) ent-Shi ketone (ent-36), Oxone, nBu4NHSO4, aq. K2CO3, Na2B4O7 buffer, pH 10.5, (CH3O)2CH2/CH3CN/H2O, rt, 61%, 3.5:1 dr; i) BF3•OEt2, CH2Cl2, −78 °C; j) Ac2O, Et3N, DMAP, CH2Cl2, rt, 25% from ent-14; k) (i) O3, NaHCO3, CH2Cl2/MeOH, −78 °C; (ii) PPh3, −78 °C to rt, 74%.