Table 3.
Incorporation of exogenous trapping nucleophiles.
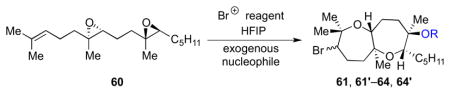
| ||||
|---|---|---|---|---|
| Entry | Nucleophile (equiv) | R | Product | Yield (%)a |
| 1 | Bu4NOAc (1.5) | Ac | 61, 61′ | 74b |
| 2 | EtOH (56) | Et | 62, 62′ | 56c |
| 3 | H2O (125) | H | 63, 63′ | 41b |
| 4 | -- | CH(CF3)2 | 64, 64′ | 33c |
| 5 | CsOH•H2O (1.5) | H | 63, 63′ | 45b,d |
Isolated as a 1:1 mixture of diastereomers in all cases.
NBS used.
Br(coll)2ClO4 used.
19% of 64, 64′ also isolated.
