Fig. 7.
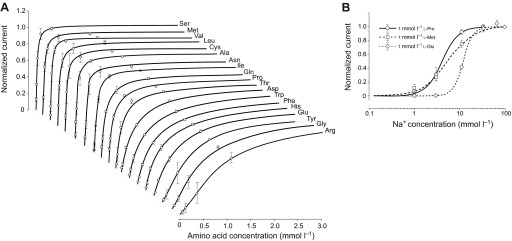
Kinetic properties of SNF-5 interaction with substrates. (A) Amino acid saturation profiles. The specified amino acids were applied in staircase concentration increments: 0.011<0.033<0.11<0.33<1.1<3.3<10 mmol l−1 prepared in 7.2 pH buffered 98 mmol l−1 Na+ medium. Graphs show only up to the 3 mmol l−1 concentrations of the specified amino acids. (B) Na+ saturation profiles with linear and logarithmic scale, respectively. The specified amino acids were applied at 1 mmol l−1 concentrations in media containing variable concentrations of Na+. Stepwise, these concentrations were: 1<3.3<10<33<98 mmol l−1, where Na+ was substituted with equimolar concentrations of N-methyl-d-glucamine (NMDG+). Data points are mean ± s.e.m. current responses normalized to maximum current in individual oocytes, for N>3 different oocytes. The data were extrapolated to fit a three-parameter sigmoidal Hill approximation f=ImaxIη/(K0.5η +Iη) without parametric constraints of Michaelis–Menten (η=1). Estimated affinities (K0.5) and apparent order of translocation events (η, Hill constant) for amino acid and Na+ substrates are summarized in Tables 2 and 3, respectively.
