Table 1.
1H NMR chemical shifts (δH), chemical shift differences (ΔδH, exo - endo), multiplicities (m), and coupling constants (J) for the endo and exo protons at C15 of various ent-beyerane derivatives
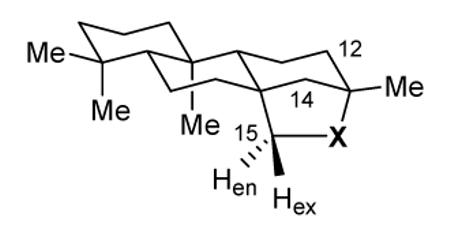
| Compound No. |
X | H15 δH (ppm) |
ΔδH (ppm) |
m | J (Hz) |
|---|---|---|---|---|---|
| 16 | C=O | endo 1.75 exo 2.68 |
0.93 | d dd |
18.6 18.6, 3.6 |
| 19 | C=NOH | endo 1.94 exo 3.02 |
1.08 | d dd |
18.4 19, 3.2 |
| 23 | NCO2Me | endoa 2.90 endob 2.97 exoa 3.89 exob 3.98 |
0.99a 1.01b |
d d dd dd |
11 11 11, 2 11, 2 |
| 12 | NH | endo 2.56 exo 3.40 |
0.84 | br d br d |
10.8 10.8 |
| 13 | N | endo 2.28 exo 3.20 |
0.92 | dd d |
12, 1.6 11.6 |
| 25a | CHOH (endo OH) |
endo NDc exo 1.85 |
NDc | NDc ddd |
NDc 14.3, 4.8, 2.6 |
| 25b | CHOH (exo OH) |
endo NDc exo 2.59 |
NDc | NDc ddd |
NDc 14.3, 7.6, 2.4 |
Major rotamer.
Minor rotamer.
ND = not determined.
