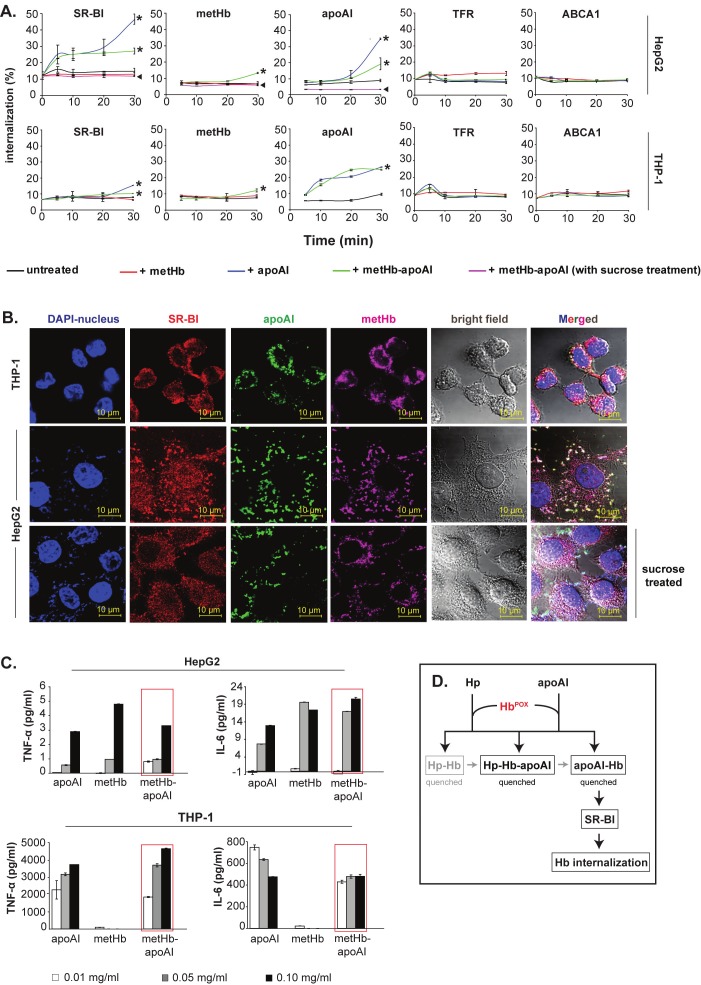
Figure 6.
SR-BI-mediated internalization of Hb-apoAI complex. (A) Quantification of the internalization of SR-BI, metHb and apoAI into hepatocytes (HepG2) and macrophages (THP-1). The % endocytosed proteins were normalized against either total biotinylated cell surface expression for the SR-BI receptor or total biotinylated proteins applied (metHb, apoAI); * indicates the internalized SR-BI, metHb or apoAI; ◄ indicates the internalization level upon treatment with 0.45 M sucrose. Two receptors, TFR and ABCA1, served as negative controls in the cells incubated with metHb and/or apoAI. (B) Immunofluorescence microscopy of internalized metHb-apoAI. The intracellular white spots in the merged images indicate co-localized proteins. (C) Cytokine production by HepG2 and THP-1 following endocytosis of metHb-apoAI. Increasing amounts of proteins (metHb and/or apoAI) were incubated with the cells for 1 h to allow endocytosis to occur. The production of TNF-α and IL-6 in the cell supernatant was determined after 24 h. The red boxes highlight the dose-dependent upregulation of TNF-α and IL-6 upon endocytosis of metHb-apoAI complex. (D) A model to illustrate the antioxidant activities of lipid-free apoAI and Hp. Cell-free oxidative Hb was rapidly captured by Hp and apoAI to form complexes in plasma, all of which quenched the redox activity of Hb (HbPOX). Following association with Hb, the apoAI further leads the internalization of Hb-apoAI complex via its recognition by SR-BI on macrophages and hepatocytes. Black color represents interactions identified in this study while grey indicates either known or speculated process.