Abstract
OBJECTIVE
To determine whether subgroups of type 1 diabetic patients with different glucose variability indices respond differently to continuous subcutaneous insulin infusion (CSII) in terms of reduced hypoglycemic events.
RESEARCH DESIGN AND METHODS
We studied 50 adults with long-standing type 1 diabetes switched to CSII because of persistently high A1C or frequent hypoglycemia despite well-managed intensive basal-bolus therapy. We compared A1C, hypoglycemic events, and glucose variability from self-monitoring of blood glucose profiles at baseline and after 6 months of CSII. Regression analysis was performed to identify predictors of response.
RESULTS
In multivariate analysis, baseline low blood glucose index (LBGI) was the best independent predictor of hypoglycemia outcome on CSII (R2 = 0.195, P = 0.0013). An ROC curve analysis demonstrated a sensitivity of 70.8% (95% CI 48.9–87.4) and specificity of 73.1% (52.2–88.4) by using the LBGI cutoff of 3.34 as predictor of reduction of hypoglycemia on CSII. By grouping patients by LBGI tertiles, we found a 23.3% reduction in hypoglycemic events (<60 mg/dL [3.3 mmol/L]) in the third tertile (range 4.18–9.34) without change in A1C (P < 0.05). Conversely, the first tertile (range 0.62–2.05) demonstrated the greatest A1C reduction, −0.99% (P = 0.00001), but with increasing hypoglycemia.
CONCLUSIONS
Baseline LBGI predicts the outcome of type 1 diabetic patients who switch to CSII in terms of hypoglycemia.
Continuous subcutaneous insulin infusion (CSII) by pump is widely used for patients with type 1 diabetes with persistently high A1C or frequent, disabling, or severe hypoglycemia, despite well-managed intensive insulin therapy with multiple daily injections (MDIs) (1,2).
For patients who fail to achieve A1C targets (3), numerous meta-analyses of randomized controlled trials support the effectiveness of CSII, with a mean decrease of 0.5% in A1C in comparison with MDI (4–13). The greatest improvements in glycemic control were in individuals with the highest A1C: patients with A1C levels ≥8.5% being treated with MDI may expect a decrease of ~1.0–1.5% with CSII (6,8,14–16).
In contrast, the ability of CSII to reduce hypoglycemic episodes remains a matter of debate. While some trials or meta-analyses showed a reduction in severe (requiring third-party assistance) (11) and nonsevere (5) hypoglycemic episodes on CSII compared with MDI, others did not (6,7,10,12) or even reported a higher rate of mild hypoglycemic events compared with MDI (13).
In everyday life, individuals with type 1 diabetes experience, on average, about two episodes of mild but symptomatic hypoglycemia per week (17). In the absence of impaired hypoglycemia awareness, mild hypoglycemia is, thus, far more frequent than severe hypoglycemia (average incidence of 1.0–1.7 episodes per year) and may exert harmful psychological effects and hamper an individual’s social life (18). These repeated events can be a limiting factor preventing improvement or maintenance of glycemic control through the deliberate avoidance of frequent episodes of low glucose levels (19,20). A recent history of hypoglycemia is also the cause of hypoglycemia-associated autonomic failure (HAAF), leading to defective glucose counterregulation and hypoglycemia unawareness and fueling the vicious cycle of recurrent hypoglycemia (20). However, while patients with frequent severe hypoglycemia may show improvement with CSII (8), clinical characteristics that could predict a reduction in overall hypoglycemia when switching from MDI to CSII are, to our knowledge, currently unknown.
Consequently, the aim of this study was to identify factors predicting mild hypoglycemic events (defined as glucose value <60 mg/dL [3.3 mmol/L]) when switching from MDI to CSII. More specifically, we tested the hypothesis that patients with the highest baseline glucose variability would be those in whom CSII reduces it the most, translating into a significant reduction of hypoglycemic events.
RESEARCH DESIGN AND METHODS
We retrospectively reviewed medical records of 69 adult patients with type 1 diabetes who were consecutively switched to CSII in our hospital because of persistently high A1C or frequent hypoglycemia despite a well-managed intensive basal-bolus therapy. Rapid-acting analogs (NovoRapid [insulin aspart]; Novo Nordisk, Bagsvaerd, Denmark, or Humalog [insulin lispro]; Eli Lilly, Indianapolis, IN) were used for meals and bolus corrections. Once- or twice-daily Lantus (insulin glargine; Sanofi, Paris, France) or Levemir (insulin detemir; Novo Nordisk, Bagsvaerd, Denmark) were mainly used for basal coverage. A few patients used NPH as basal insulin. Patients were asked to perform at least four capillary blood glucose determinations per day and to keep a log book of insulin and glucose data. They were also taught to document hypoglycemia by obtaining a capillary glucose determination whenever possible.
Initiation of CSII was performed during a short hospitalization. A Medtronic pump (Paradigm or Veo; Medtronic, Minneapolis, MN) with insulin lispro or insulin aspart was used for all patients. During the initial 3- to 5-day inpatient period, a diabetes nurse and a dietitian conducted several education sessions about insulin adjustments, basic carbohydrate counting, and pump management. Basal rates were determined as a function of insulin dosage on MDI and were adjusted during the inpatient period dependent on daily eight-point glucose profiles. Patients returned thereafter to the outpatient clinic after 6 weeks, 3 months, and 6 months for self-monitoring of blood glucose (SMBG) profile review and education, insulin dosage adjustments, blood sampling, and clinical evaluation. A1C was assessed by a Diabetes Control and Complications Trial standardized cation-exchange chromatographic assay (nondiabetic reference 4–6%) and measured from venous blood samples during the hospitalization for CSII initiation and then at 3- and 6-month visits. The hospital’s ethics committee approved the study.
Glucose profiles and variability
SMBG data were downloaded from patients’ glucose meters at each visit and converted to raw data for further calculations. When raw glucose data were not available, printed glucose profiles were scanned and digitized with Engauge Digitizer software (http://digitizer.sourceforge.net) to retrieve numeric data. Data from a 1-month period preceding as close as possible (and no more than 3 months from) the initial hospitalization and the 6-month visit were used for calculation of SMBG frequency, glucose mean, documented hypoglycemic events, and glucose variability.
We determined glucose variability with several SMBG-derived indices, since there is no gold standard method and each of them may be sensitive to different aspects of variability (21). In addition to SD, we calculated the percent coefficient of variation (%CV = 100 × SD/mean), the low blood glucose index (LBGI), the high blood glucose index (HBGI), and the average daily risk range (ADRR), which amalgamates information similar to LBGI and HBGI with a supplementary level of aggregation (21–25). ADRR was computed only when SMBG data contained at least three readings/day for at least 14 days during the 1-month period to guarantee its reliability (24).
Hypoglycemia
Documented low-glucose events (symptomatic and asymptomatic) <70 mg/dL (3.9 mmol/L), 60 mg/dL (3.3 mmol/L), and 50 mg/dL (2.8 mmol/L) were counted from SMBG values by an algorithm encoded in Microsoft Excel (version 2010; Microsoft, Redmond, WA). To avoid taking into account multiple glucose determinations of the same event, we used an algorithm that considered values in a row below the cutoff as a single episode. Hypoglycemic events were reported as events per patient per week, and change in number of events (∆hypoglycemia) was calculated as the difference in number of episodes per patient per week <60 mg/dL (3.3 mmol/L) from MDI to CSII and expressed in events per patient per week or relative percentage of change.
Statistical analysis
Normal distribution was assessed for each variable using the Shapiro-Wilk test. Results are expressed as means ± SD for parametric data and median (interquartile range) for nonparametric data unless otherwise stated. Comparisons between groups were performed using two-tailed paired Student t, Wilcoxon, or Mann-Whitney U tests as appropriate. Linear regression analysis was used to detect predictors for hypoglycemic events and A1C outcomes on CSII. Variables tested in the univariate analysis were age, diabetes duration, baseline A1C, mean blood glucose, number of low glucose events (<70, <60, and <50 mg/dL), SMBG measurements per day, SD of blood glucose, %CV of blood glucose, LBGI, HBGI, and ADRR. Variables correlated with the respective outcome at P < 0.20 were entered in a stepwise multivariate regression analysis. Other correlations were analyzed by means of Spearman correlation test. For intertertile A1C comparisons, we used one-way unpaired ANOVA with post hoc Tukey test when statistically differences were found. For intertertile comparisons of hypoglycemic events, LBGI, HBGI, and ADRR, we used the Kruskal-Wallis test with post hoc Mann-Whitney U testing when statistically significant differences were found. P values <0.05 were considered statistically significant. All statistical calculations were performed with the StatEL software (version 2.6, www.adscience.eu) add-on for Microsoft Excel (version 2010; Microsoft). Regression lines and receiver operating characteristic (ROC) curves were generated by MedCalc software (version 12.3, www.medcalc.org).
RESULTS
Thirteen patients were not included in the analysis because of incomplete baseline SMBG data. One patient was excluded because she became pregnant <6 months after CSII initiation, and five other patients were excluded because they did not have sufficient SMBG data after the switch to CSII. The final cohort thus consisted of 50 patients aged 21–71 years with long-standing type 1 diabetes (mean ± SD 19.9 ± 10.4 years) and indices of high glucose variability (see Table 1). The primary indication for CSII was for persistent hyperglycemia in 26 patients (52%), frequent or severe hypoglycemia in 18 patients (36%), and mixed (both hyperglycemia and hypoglycemia) in 6 patients (12%).
Table 1.
Characteristics of study patients
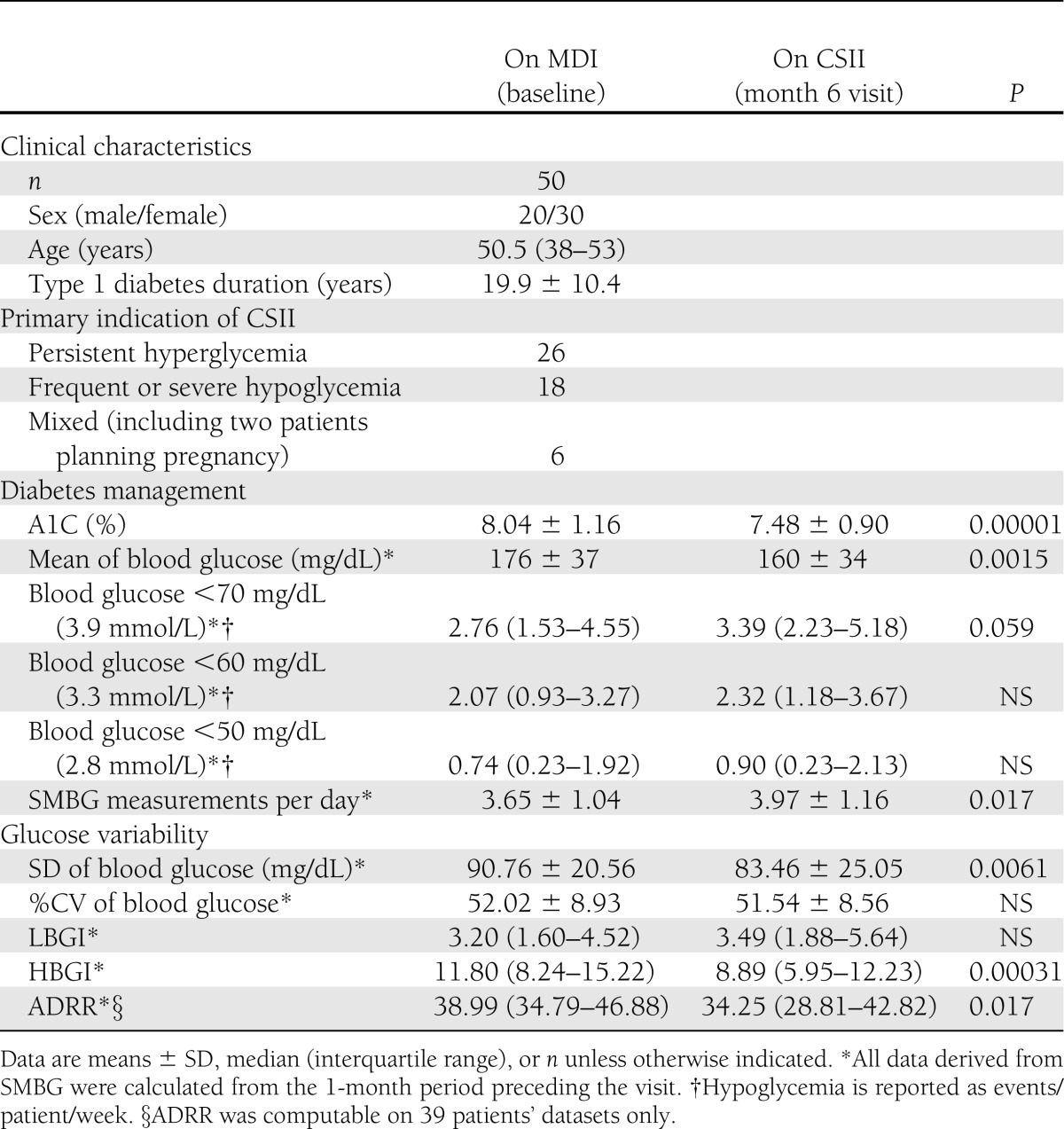
After 6 months’ treatment with CSII (Table 1), there was a significant reduction in mean ± SD blood glucose—from 176 ± 37 mg/dL on MDI to 160 ± 34 mg/dL with CSII (P = 0.0015)—which is reflected by the significant improvement in A1C, 8.04 ± 1.16% on MDI vs. 7.48 ± 0.90% on CSII: a decrease of 0.56% (P = 0.00001). This significant improvement in A1C was not accompanied by an increased rate of hypoglycemic events (events/patient/week) <50 mg/dL (2.8 mmol/L), median 0.74 (interquartile range 0.23–1.92) vs. 0.90 (0.23–2.13) (P = NS), or <60 mg/dL (3.3 mmol/L), 2.07 (0.93–3.27) vs. 2.32 (1.18–3.67) (P = NS). However, there was a trend toward an increase in documented events <70 mg/dL (3.9 mmol/L): 2.76 (1.53–4.55) vs. 3.39 (2.23–5.18) (P = 0.059). There was also a slight but significant increase in mean SMBG measurements: 3.65 ± 1.04 on MDI vs. 3.97 ± 1.16 on CSII (P = 0.017). Several of our patients experienced one or more episodes of severe hypoglycemia (data not shown). Because of the short follow-up time of this study, the number of these events was too low to be analyzed accurately.
We observed a decrease in glucose variability assessed by ADRR after 6 months on CSII: median 38.99 (interquartile range 34.79–46.88) on MDI vs. 34.25 (28.81–42.82) on CSII (P = 0.017). When LBGI and HBGI, which are selectively sensitive to low and high glucose peaks, are considered, it appears that this benefit was entirely the result of the reduction of high blood glucose peaks, as HBGI decreased from 11.80 (8.24–15.22) to 8.89 (5.95–12.23) (P = 0.00031) while LBGI did not change. Glucose SD decreased significantly: 90.76 ± 20.56 vs. 83.46 ± 25.05 mg/dL (P = 0.0061), but %CV did not change. At baseline, LBGI was higher in patients switching to CSII because of hypoglycemia (median 3.39 [interquartile range 2.54–4.53]) in comparison with whose switching for sustained hyperglycemia (2.58 [1.46–4.52]), but given the small number of patients in each group, this did not reach significance (P = 0.068).
Multiple regression analysis and ROC curves of predictors of outcome
To identify potential predictive factors of glycemic outcome, we performed a univariate regression analysis: first against reduction of hypoglycemic events (∆hypoglycemia) and then against lowering of A1C level (∆A1C). Correlations at the P < 0.2 level with the corresponding outcome are shown in Table 2.
Table 2.
Univariate and multivariate analysis of predictors of A1C and hypoglycemia outcomes on CSII
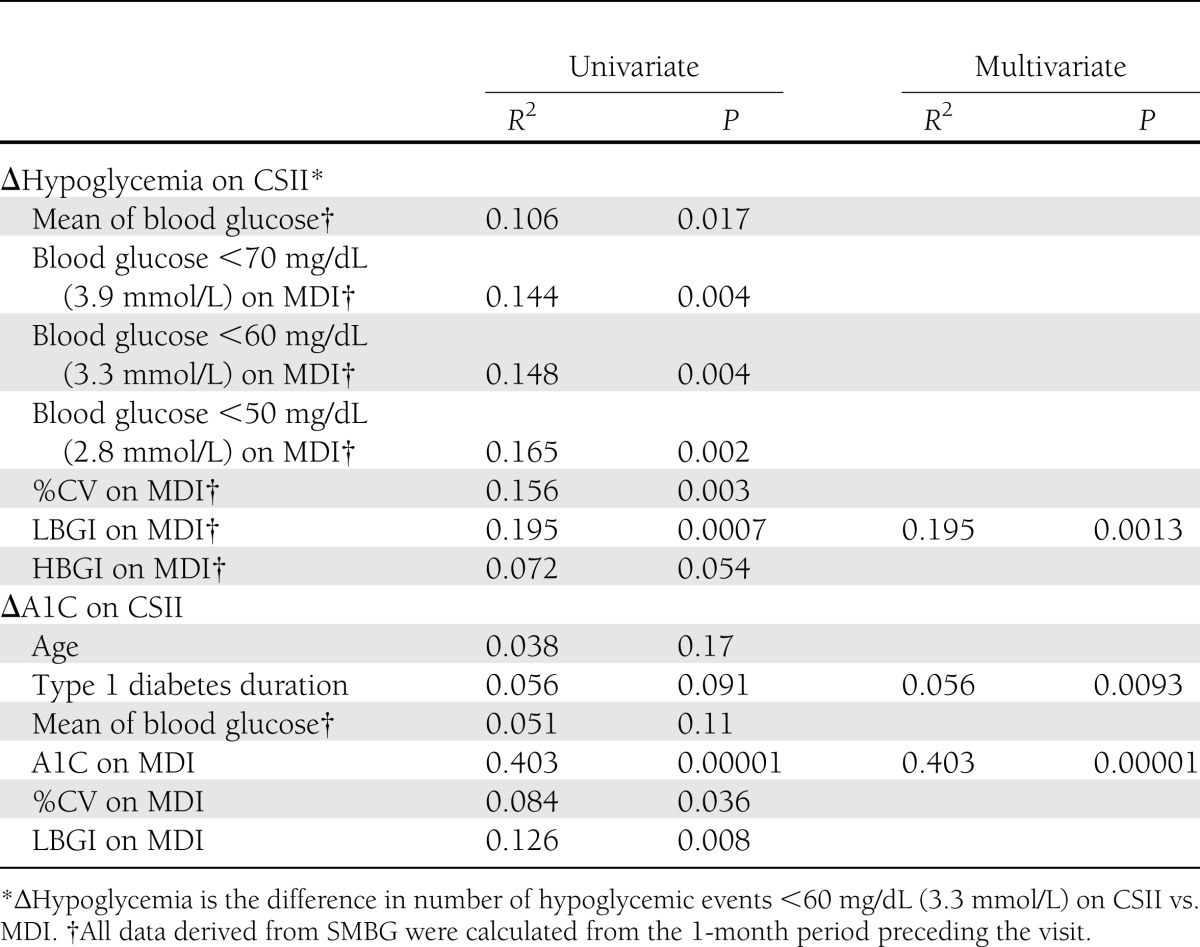
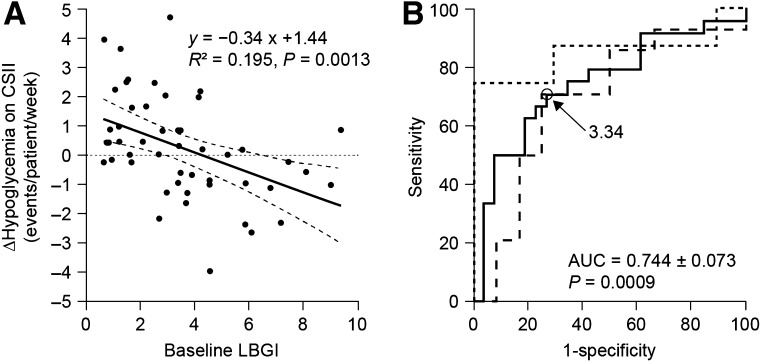
Baseline LBGI and number of events <50 mg/dL were the best correlates with the ∆hypoglycemia outcome (R2 = 0.195 and 0.165, respectively). In the multivariate analysis, only baseline LBGI correlated independently with ∆hypoglycemia (R2 = 0.195, P = 0.0013). The regression line is shown in Fig. 1A. Correlation was similar when the 70 mg/dL (3.9 mmol/L) threshold for ∆hypoglycemia was used. However, when the reduction of hypoglycemic events <50 mg/dL (2.8 mmol/L) was taken as outcome, the best independent correlate was no longer LBGI but, rather, the baseline number of events <50 mg/dL (2.8 mmol/L) (R2 = 0.340, P = 0.00001) (Supplementary Table).
Figure 1.
Prediction of ∆hypoglycemia outcome on CSII by LBGI. A: Regression line of baseline LBGI for changes in hypoglycemic events (<60 mg/dL [3.3 mmol/L]) on CSII. Dotted lines are 95% CI. B: ROC curve of LBGI as predictor of a reduction of hypoglycemic events on CSII in the entire cohort (solid line) and in the subgroups switching to CSII because of hyperglycemia (dashed line) and hypoglycemia (dotted line).
We then constructed an ROC curve to determine the optimal LBGI cutoff for predicting a reduction of hypoglycemic events (∆hypoglycemia <0). The area under the curve (AUC) was 0.744 ± 0.073 (P = 0.0009 in comparison with area = 0.5), and the LBGI value of 3.34 gives the best compromise of sensitivity, 70.8% (95% CI 48.9–87.4), and specificity, 73.1% (52.2–88.4), in our cohort (Fig. 1B). When analyzing subgroups by function of the pump indication, we found an ROC curve with an AUC of 0.690 ± 0.113 (P = 0.09) for patients with persistent hyperglycemia on MDI and of 0.85 ± 0.116 (P = 0.0026) for patients with frequent or severe hypoglycemia. In this group, sensitivity reached 75% (34.9–96.8) and specificity 100% (69.2–100.0), but the number of patients in each subgroup was insufficient to compare the curves between them (Fig. 1B).
Several factors significantly correlated with the change in A1C with CSII, the best correlates being baseline A1C level (R2 = 0.403, P = 0.00001) and LBGI on MDI (R2 = 0.126, P = 0.008). In the multivariate analysis, only baseline A1C level (R2 = 0.403, P = 0.00001) and, to a lesser extent, diabetes duration (R2 = 0.056, P = 0.0093) were independently correlated with ∆A1C. The regression line for A1C is displayed in Supplementary Fig. 1A.
Baseline A1C also correlates negatively with baseline LBGI (R2 = 0.216, P = 0.0011) (Supplementary Fig. 1B). Given that LBGI correlates with the ∆hypoglycemia outcome, it would be intuitive to postulate that baseline A1C also correlates with the ∆hypoglycemia outcome, but we did not find such correlation (Supplementary Table 1).
Analysis by LBGI tertiles
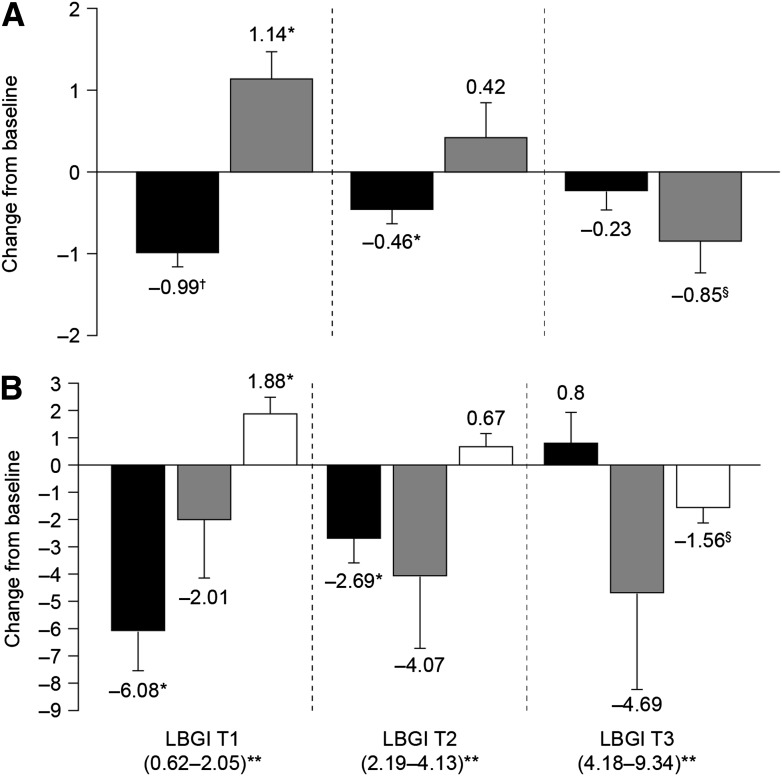
To display the combined correlations between baseline LBGI and ∆hypoglycemia, baseline A1C and baseline LBGI, and baseline A1C and ∆A1C, we compared the outcome of the patients divided into three equal groups sorted by ascending value of baseline LBGI (tertiles). The third tertile (median 5.84 [range 4.18–9.34]) was associated with a reduction in hypoglycemia of 0.85 ± 1.48 events/patient/week (or a 23.3% decrease) when treated with CSII (P < 0.05 vs. MDI). In the first tertile (1.20 [0.62–2.05]), hypoglycemia was not reduced but increased to 1.14 ± 1.38 events/patient/week (P < 0.01 vs. MDI, P < 0.003 for intertertile comparison) (Fig. 2A). In accordance with the change in hypoglycemia, patients with the highest baseline LBGI (third tertile) had significantly reduced LBGI value when switching to CSII, –1.56 ± 2.21 (P < 0.05 vs. MDI), while in the first tertile LBGI increased by 1.88 ± 2.44 (P < 0.01 vs. MDI, P < 0.0007 for intertertile comparison) (Fig. 2B).
Figure 2.
Evolution of diabetes control and glucose variability at 6 months after switching to CSII, categorized by tertiles of baseline LBGI. A: Absolute differences (CSII − baseline) in mean A1C expressed as percentage (black bars) and in hypoglycemic events expressed as events/patient/week (gray bars). B: Absolute differences (CSII − baseline) in mean HBGI (black bars), ADRR (gray bars), and LBGI (white bars). *P = 0.01 vs. MDI, †P = 0.00001 vs. MDI, §P < 0.05 vs. MDI. Intertertile comparisons significant for mean A1C changes (P < 0.014 by ANOVA, post hoc Tukey showing T3 > T1), changes in hypoglycemic events (P < 0.0029 by Kruskal-Wallis, post hoc Mann-Whitney showing T3 < T1), changes in HBGI (P < 0.0008 by Kruskal-Wallis, post hoc Mann-Whitney showing T1 < T2 and T2 < T3), and change in LBGI (P < 0.0007 by Kruskal-Wallis, post hoc Mann-Whitney showing T1 = T2 and T2 > T3). Errors bars are SEM. **Numbers in parentheses are the range of the tertile.
Consistent with the LBGI and A1C correlation, the first tertile was the one with the highest baseline A1C, 8.69 ± 1.08 vs. 7.39 ± 1.02% for the third tertile (P < 0.004 for intertertile comparison), and it benefitted from the best A1C reduction, −0.99 ± 0.64% (P = 0.00001 vs. MDI, P < 0.014 for intertertile comparison) (Fig. 2A). The reduction in A1C was also accompanied by the greatest HBGI reduction: –6.08 ± 0.64 (P < 0.01 vs. MDI, P < 0.0008 for intertertile comparison) (Fig. 2B).
Finally, patients from the middle tertile (median 3.34 [range 2.19–4.13]) experienced a smaller but significant improvement in A1C, −0.46 ± 0.66% vs. MDI (P = 0.01), without significant change in hypoglycemia.
CONCLUSIONS
We describe here a cohort of 50 patients with a long duration of type 1 diabetes who switched from MDI to CSII. Their mean number of events <60 mg/dL (3.3 mmol/L) corresponds well to the number of symptomatic hypoglycemic events experienced by an average patient with type 1 diabetes (17), and their overall outcome in terms of A1C and hypoglycemia was similar to that usually reported in the literature: A1C was reduced by ~0.5%, without a significant increase in hypoglycemia. In accordance with previous continuous glucose monitoring system studies, we also observed significant reductions in glucose variability (assessed by ADRR) and hyperglycemic peaks (assessed by HBGI) after switching to CSII (26,27).
Our main finding was that baseline LBGI derived from a 1-month period of systematic SMBG predicts hypoglycemia outcome on CSII. By virtue of choosing an appropriate cutoff, the sensitivity and specificity of LBGI to predict a reduction in hypoglycemic events were both >70%, and these scores might be even better in the subgroup of patients switched to the pump for frequent or severe hypoglycemia.
We also show that LBGI, which is sensitive to both the magnitude and frequency of low glucose events, is a better predictor of the hypoglycemia outcome than baseline hypoglycemic event rates alone. Documenting hypoglycemic events in a retrospective study has limitations because there is a risk of underreporting. However, this situation corresponds to real-life, where the assessment of hypoglycemic events during routine visits is largely retrospective. Moreover, LBGI calculations have been validated with SMBG collections of no more than 50 readings over a 2- to 3-week period (28).
Although we were unable to show an overall improvement of hypoglycemia with CSII, LBGI divided our population into two groups (first and third tertiles) with opposite outcomes. Patients with the lowest LBGI values experienced improvements in HBGI and A1C but with increased LBGI and more hypoglycemia. By contrast, patients with the highest LBGI values improved their LBGI and experienced a decreased number of hypoglycemic events but with no significant improvement in A1C or HBGI. This finding may explain the conflicting results in the literature regarding hypoglycemia outcome with CSII treatment. Indeed, in the absence of a systematic determination of baseline LBGI before switching to CSII, a small imbalance in favor of patients with higher LBGI values may lead to a better global hypoglycemia outcome and vice versa.
Previous data support our results. Patients with type 1 diabetes with the highest rates of severe hypoglycemia on MDI experience the largest reduction in severe hypoglycemic episodes when switched to CSII (8). Given that LBGI correlates with risk of severe hypoglycemia (23,28), it is likely that this high-risk population also has high LBGI values, which would be consistent with our results showing that patients with the highest LBGI display the greatest reduction in hypoglycemic events.
We confirmed that baseline A1C correlates well with A1C reduction with CSII (6,8,14–16) but, on the other hand, provides no indication about response to hypoglycemia, although baseline A1C correlates (negatively) with baseline LBGI. As LBGI is computed from actual glucose values and not from amplitude of glucose excursions, an SMBG profile with a higher glucose mean will be associated with a lower LBGI than another profile with the same intrinsic glucose variability but with lower mean glucose. We thus hypothesize that high A1C may lower LBGI in a way not directly linked to the intrinsic variability and the hypoglycemia risk.
In summary, LBGI derived from SMBG data is, as far as we know, the first identified predictor of mild hypoglycemia outcome when switching to CSII. Patients with the highest LBGI values experience the greatest reduction of hypoglycemia, while those with the lowest LBGI values and the highest starting A1C level obtain the greatest lowering of A1C on CSII. We are now undertaking a prospective trial using both SMBG and continuous glucose monitoring system data to confirm these findings.
Acknowledgments
L.C. has served on advisory boards for Medtronic Benelux. No other potential conflicts of interest relevant to this article were reported.
L.C. designed the study, researched and analyzed data, and wrote the manuscript. C.A.-E. researched data and contributed to the discussion. B.C. contributed to the discussion. L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the “Congrès de la Société Francophone du Diabète 2013,” Montpellier, France, 26–29 March 2013.
The authors thank J.-F. Vanderijst and V. Col (Clinique Saint-Pierre, Ottignies, Belgium) for providing patients and N. Hussin, G. Van Braekel, and D. Antoine (ULB-Erasme Hospital) for organizing and performing the CSII educational sessions. The authors also thank C. Melot (ULB-Erasme Hospital) for assistance with statistics and L. Southey and H. Marshall of Watermeadow Medical for writing and editorial assistance, funded by ULB-Erasme Hospital.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-2058/-/DC1.
References
- 1.Pickup JC. Management of diabetes mellitus: is the pump mightier than the pen? Nat Rev Endocrinol 2012;8:425–433 [DOI] [PubMed] [Google Scholar]
- 2.Hanaire H. External insulin pump treatment in the day-to-day management of diabetes: benefits and future prospectives. Diabetes Metab 2011;37(Suppl. 4):S40–S47 [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(Suppl. 1):S11–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pickup JC, Mattock MB, Kerry S. Glycaemic control with continuous subcutaneous insulin infusion compared with intensive insulin injections in patients with type 1 diabetes: meta-analysis of randomised controlled trials. BMJ 2002;324:705–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weissberg-Benchell J, Antisdel-Lomaglio J, Seshadri R. Insulin pump therapy: a meta-analysis. Diabetes Care 2003;26:1079–1087 [DOI] [PubMed] [Google Scholar]
- 6.Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care 2004;27:2590–2596 [DOI] [PubMed] [Google Scholar]
- 7.Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941–951 [DOI] [PubMed] [Google Scholar]
- 8.Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in Type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med 2008;25:765–774 [DOI] [PubMed] [Google Scholar]
- 9.Jacobsen IB, Henriksen JE, Hother-Nielsen O, Vach W, Beck-Nielsen H. Evidence-based insulin treatment in type 1 diabetes mellitus. Diabetes Res Clin Pract 2009;86:1–10 [DOI] [PubMed] [Google Scholar]
- 10.Fatourechi MM, Kudva YC, Murad MH, Elamin MB, Tabini CC, Montori VM. Clinical review: Hypoglycemia with intensive insulin therapy: a systematic review and meta-analyses of randomized trials of continuous subcutaneous insulin infusion versus multiple daily injections. J Clin Endocrinol Metab 2009;94:729–740 [DOI] [PubMed] [Google Scholar]
- 11.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev 2010:CD005103. [DOI] [PubMed] [Google Scholar]
- 12.Monami M, Lamanna C, Marchionni N, Mannucci E. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in type 1 diabetes: a meta-analysis. Acta Diabetol 2010;47(Suppl. 1):77–81 [DOI] [PubMed] [Google Scholar]
- 13.Yeh HC, Brown TT, Maruthur N, et al. Comparative effectiveness and safety of methods of insulin delivery and glucose monitoring for diabetes mellitus: a systematic review and meta-analysis. Ann Intern Med 2012;157:336–347 [DOI] [PubMed] [Google Scholar]
- 14.Lepore G, Dodesini AR, Nosari I, Trevisan R. Age and A1C are important clinical predictors of continuous subcutaneous insulin infusion efficacy in type 1 diabetic patients. Diabetes Care 2005;28:1834–1835 [DOI] [PubMed] [Google Scholar]
- 15.Retnakaran R, DeVries JH, Hanaire-Broutin H, Heine RJ, Melki V, Zinman B. Continuous subcutaneous insulin infusion versus multiple daily injections: modeling predicted benefits in relationship to baseline A1c. Diabetes Care 2005;28:1835–1836 [DOI] [PubMed] [Google Scholar]
- 16.Partridge H, Kerr D. Advantages of insulin pump therapy. Diabet Med 2009;26:1306. [DOI] [PubMed] [Google Scholar]
- 17.Frier BM. The incidence and impact of hypoglycemia in type 1 and type 2 diabetes. International Diabetes Monitor 2009;21:210–218 [Google Scholar]
- 18.Frier BM. Morbidity of hypoglycemia in type 1 diabetes. Diabetes Res Clin Pract 2004;65(Suppl. 1):S47–S52 [DOI] [PubMed] [Google Scholar]
- 19.McCrimmon RJ, Frier BM. Hypoglycaemia, the most feared complication of insulin therapy. Diabete Metab 1994;20:503–512 [PubMed] [Google Scholar]
- 20.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes 2008;57:3169–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodbard D. Clinical interpretation of indices of quality of glycemic control and glycemic variability. Postgrad Med 2011;123:107–118 [DOI] [PubMed] [Google Scholar]
- 22.Rodbard D. Optimizing display, analysis, interpretation and utility of self-monitoring of blood glucose (SMBG) data for management of patients with diabetes. J Diabetes Sci Tech 2007;1:62–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovatchev BP, Cox DJ, Gonder-Frederick LA, Young-Hyman D, Schlundt D, Clarke W. Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 24.Kovatchev BP, Otto E, Cox D, Gonder-Frederick L, Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care 2006;29:2433–2438 [DOI] [PubMed] [Google Scholar]
- 25.McCall AL, Kovatchev BP. The median is not the only message: a clinician perspective on mathematical analysis of glycemic variability and modeling in diabetes mellitus. J Diabetes Sci Tech 2009;3:3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruttomesso D, Crazzolara D, Maran A, et al. In Type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med 2008;25:326–332 [DOI] [PubMed] [Google Scholar]
- 27.Chimenti EM, de la Morena LH, Vaquero PM, Sáez-de-Ibarra L, Domínguez MG, Sánchez LF. Assessing glycaemic variability with continuous glucose monitoring system before and after continuous subcutaneous insulin infusion in people with Type 1 diabetes. Diabetes Res Clin Pract 2010;90:e57–e59 [DOI] [PubMed] [Google Scholar]
- 28.Cox DJ, Kovatchev BP, Julian DM, et al. Frequency of severe hypoglycemia in insulin-dependent diabetes mellitus can be predicted from self-monitoring blood glucose data. J Clin Endocrinol Metab 1994;79:1659–1662 [DOI] [PubMed] [Google Scholar]