Abstract
OBJECTIVE
We examined if chronic cannabis smoking is associated with hepatic steatosis, insulin resistance, reduced β-cell function, or dyslipidemia in healthy individuals.
RESEARCH DESIGN AND METHODS
In a cross-sectional, case-control study, we studied cannabis smokers (n = 30; women, 12; men, 18; 27 ± 8 years) and control subjects (n = 30) matched for age, sex, ethnicity, and BMI (27 ± 6). Abdominal fat depots and intrahepatic fat content were quantified by magnetic resonance imaging and proton magnetic resonance spectroscopy, respectively. Insulin-sensitivity indices and various aspects of β-cell function were derived from oral glucose tolerance tests (OGTT).
RESULTS
Self-reported cannabis use was: 9.5 (2–38) years; joints/day: 6 (3–30) [median (range)]. Carbohydrate intake and percent calories from carbohydrates, but not total energy intake, were significantly higher in cannabis smokers. There were no group differences in percent total body fat, or hepatic fat, but cannabis smokers had a higher percent abdominal visceral fat (18 ± 9 vs. 12 ± 5%; P = 0.004). Cannabis smokers had lower plasma HDL cholesterol (49 ± 14 vs. 55 ± 13 mg/dL; P = 0.02), but fasting levels of glucose, insulin, total cholesterol, LDL cholesterol, triglycerides, or free fatty acids (FFA) were not different. Adipocyte insulin resistance index and percent FFA suppression during an OGTT was lower (P < 0.05) in cannabis smokers. However, oral glucose insulin sensitivity index, measures of β-cell function, or incretin concentrations did not differ between the groups.
CONCLUSIONS
Chronic cannabis smoking was associated with visceral adiposity and adipose tissue insulin resistance but not with hepatic steatosis, insulin insensitivity, impaired pancreatic β-cell function, or glucose intolerance.
Cannabinoid receptors (CB1R and CB2R) and their endogenous ligands, the endocannabinoids, play an important role in regulating energy balance, appetite, insulin sensitivity, pancreatic β-cell function, and lipid metabolism (1–4). Endocannabinoids (anandamide and 2-arachidonoyl glycerol) and CB1Rs are present in peripheral tissues involved in energy homeostasis, such as adipose tissue, liver, skeletal muscle, and pancreas (1,2). CB1R activation promotes lipogenesis in the liver and adipose tissue (1,2,5), reduces insulin responsiveness in skeletal muscle (3), and impairs insulin action and secretion in pancreatic β-cells (4). Consistent with these findings, clinical interventional trials suggest that CB1R antagonism reduces body weight, improves dyslipidemia, and attenuates insulin resistance in humans (6,7).
Δ9-Tetrahydrocannabinol (Δ9-THC), the primary psychoactive component of Cannabis sativa, activates peripheral and central CB1Rs (2). Acute treatment of healthy human volunteers with cannabis induces glucose intolerance (8,9). Similarly, short-term (13 days) marijuana smoking increases appetite, food intake, and body weight in healthy men (10). In individuals with chronic hepatitis C, daily marijuana abuse was associated with a higher risk for hepatic steatosis (11). These findings from animal and human studies suggest that chronic cannabis use and CB1R activation may negatively affect metabolic actions of insulin and facilitate hepatic steatosis. Cannabis (marijuana) is the most commonly used illicit drug in the U.S. with ∼17 million current users (12). Despite such widespread use, relatively little is known about the metabolic effects associated with chronic cannabis use. To that end, in a cross-sectional, case-control study, we examined if chronic cannabis smoking is associated with hepatic steatosis, insulin resistance, reduced β-cell function, and dyslipidemia in healthy individuals.
RESEARCH DESIGN AND METHODS
Study design and study subjects
This cross-sectional, case-control study was conducted at the Clinical Research Center, National Institutes of Health (NIH), in Bethesda, MD (ClinicalTrials.gov Identifier: NCT00428987) and the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU) in Baltimore, MD. The study protocol was approved by the NIH Central Nervous System Institutional Review Board and Institutional Review Boards of the National Institute of Diabetes and Digestive and Kidney Diseases-National Institute of Arthritis and Musculoskeletal and Skin Diseases, and all procedures followed were in accordance with institutional guidelines. Written informed consent was obtained from all subjects. Adults who smoked cannabis at least 4 days per week for the last 6 months, with a self-reported history of cannabis smoking for at least 1 year, and not seeking active treatment were recruited by the National Institute on Drug Abuse. Exclusion criteria were dependence on drugs other than nicotine, caffeine, and cannabis within the past 6 months of screening visit; history of intravenous illegal drug use; alcohol intake of more than five drinks per day on ≥3 days in a week (>15 drinks/week); history of hepatitis B or C or current hepatitis A, B, or C; history of diabetes (type 1 or type 2), polycystic ovary disease, or other conditions that may confound study outcomes; professional or collegiate athletes or active participation in >60 min/day of vigorous exercise; use of prescribed or over-the-counter or herbal/alternative medications/preparations with effects on glucose and lipid metabolism; and pregnancy.
Cannabis smokers underwent history and physical examination as well as laboratory tests of blood and urine to ensure that they were free of current somatic and psychiatric illness and did not currently abuse drugs other than cannabis (or nicotine or alcohol). Eligible participants were admitted to the BPRU, where they were continuously monitored and not allowed to leave the unit or receive visitors during their stay to exclude illicit drug use. Following an overnight stay at BPRU, subjects were transported to the NIH Clinical Center for metabolic studies and imaging studies. After the study procedures, the subjects were transported back to BPRU. Control subjects with no prior history of cannabis use and with negative urine screens for illicit drug use were recruited by National Institute of Diabetes and Digestive and Kidney Diseases in Bethesda, MD. Control subjects were matched with cannabis smokers for sex, ethnicity, age (± 5 years), and BMI (± 2 kg/m2).
Study procedures
Dietary intake and dietary quality.
A 24-h dietary recall was used for evaluation of total daily energy intake (13). The Healthy Eating Index (HEI) 2005 was used to assess dietary quality. HEI is based on the food patterns in the 2005 Dietary Guidelines for Americans (14). The score is a composite measure of 12 energy-adjusted components, including, total grains, whole grains, total fruit, whole fruit, total vegetables, dark green and orange vegetables and legumes, milk, meat and beans, oils, saturated fat, sodium, and calories from solid fats, alcoholic beverages, and added sugars. HEI scores range from 0–100, with a higher score indicating a better-quality diet.
Assessment of body composition, adipose tissue distribution, and hepatic fat content.
Body weight was measured using a digital balance (Scale-Tronix 5702; Scale-Tronix, Carol Stream, IL). Body composition was measured by dual-energy X-ray absorptiometry with a Lunar iDXA scanner (GE Healthcare, Madison WI). Subcutaneous and visceral adipose tissue volumes were assessed at the L4–L5 spine level by magnetic resonance imaging using manual image segmentation on a T1-weighted turbo-spin-echo image as previously described (15). Hepatic fat content was measured with single volume magnetic resonance spectroscopy (MRS) in an 8 ml volume in the posterior right lobe of the liver as described previously (16).
Oral glucose tolerance test.
Each subject underwent 75-g oral glucose tolerance test (OGTT) after a 10-h overnight fast. Samples for determination of plasma glucose, insulin, C-peptide, free fatty acids (FFA), and various incretin concentrations were drawn at −10, 0, 15, 30, 45, 60, 90, 120, and 180 min relative to the glucose load. Plasma was obtained from blood samples by centrifugation and immediately frozen on dry ice for storage at − 80°C.
Laboratory assays.
Routine assays for serum lipids, plasma glucose, insulin, and hemoglobin A1c (A1C) were performed in the Department of Laboratory Medicine at the Clinical Center, NIH. FFA were measured with a Wako HR Series NEFA-HR kit (Wako Diagnostics, Wako Chemicals USA, Inc., Richmond, VA) and run on a COBAS FARA-II analyzer (Roche Diagnostics, Indianapolis, IN). Adiponectin, leptin, total peptide YY (PYY), active glucagon-like peptide-1 (GLP-1), and acyl and desacyl ghrelin levels were measured in plasma by ELISA.
Modeling and calculations.
Quantitative Insulin Sensitivity Check Index (QUICKI), Oral Glucose Insulin Sensitivity (OGIS), and Hepatic Insulin Resistance Index (HIRI) were used as surrogate measures of glucose insulin sensitivity/resistance (17–19). QUICKI was calculated as defined previously from fasting glucose and insulin values (20). QUICKI = 1/(log[I0] + log[G0]), where I0 is fasting insulin (μU/mL) and G0 is fasting glucose (mg/dL). OGIS was calculated from OGTT using the 3-h OGIS equation (18). HIRI was calculated as the product of total area under curve (AUC) for glucose and insulin during the first 30 min of the OGTT (19). The trapezoidal method was used to calculate AUC. HIRI = (G 0–30[AUC] × I 0–30[AUC]). Adipose tissue sensitivity to the antilipolytic actions of insulin were assessed by the adipocyte insulin resistance index (AIRI) (21) and the degree of suppression of plasma FFA during an OGTT (22). AIRI is the product of the fasting plasma insulin and fasting plasma FFA (log-transformed) concentrations. Suppression of lipolysis during an OGTT was assessed as FFAAUC adjusted for mean plasma insulin concentrations and percent maximal suppression of FFA levels.
β-Cell glucose sensitivity, rate sensitivity, and the potentiation factor were calculated with the model as described previously (23). In addition, insulinogenic index (IGI) was derived from plasma insulin and glucose concentrations during an OGTT as previously described (24).
Statistical analyses.
The primary outcome measures were differences in insulin sensitivity and hepatic fat content between age-, sex-, ethnicity-, and BMI-matched chronic cannabis smokers and control subjects. There were no prior studies examining the effects of chronic cannabis use on insulin sensitivity or hepatic fat content in healthy individuals. In a study of hepatic steatosis (assessed by biopsy) in hepatitis C virus (HCV)–infected individuals, 33% of daily cannabis users had marked steatosis compared with 16% of nonusers (odds ratio of 2.1) (11). In healthy individuals, we expected the effects of chronic cannabis smoking on hepatic fat content to be larger than observed in HCV-infected individuals. A sample size of n = 60, with n = 30 in each cohort was sufficient to provide 80% power with α = 0.05 in detecting a standardized difference of ∼0.75 in hepatic fat content (estimated SD, 7%) (16). Similarly, a sample size of n = 60 was sufficient to detect a standardized difference of ∼1.0 in insulin sensitivity by QUICKI (estimated SD, 0.037) (20) and OGIS (estimated SD, 60 mL · min−1 · m−2) (18) with a power of 90% and α = 0.05. The presence of skewed data were evaluated by visual inspection of quantile–quantile plots, stem and leaf plots, or box plots and verified by the Shapiro-Wilk test for normal distribution. After testing data for normality, we used Student unpaired t, paired t, or appropriate nonparametric tests for evaluation of differences between the groups. Because of the exploratory nature of this study, no adjustments were made for multiple comparisons, and values of P < 0.05 were considered to represent statistical significance. The statistical software StatsDirect version 2.7.2 (Chershire, U.K.) was used for data analysis.
RESULTS
Clinical characteristics, cannabis use, and energy intake of study subjects
This study included age-, sex-, race-, and BMI-matched chronic cannabis smokers (n = 30) and control subjects (n = 30). The median age of cannabis smokers was 25 years (25th–75th percentile: 21–28 years). Age at first cannabis smoking was 15 ± 3 years (range, 11–20). Participants smoked an average of 10 ± 8 joints per day (range 3–30) for 12 ± 9 years (range, 2–38). The majority of cannabis smokers in our cohort were African American (73%) and men (60%) (Table 1). The median BMI of the cannabis smokers was 26 kg/m2 (range, 19–42). The participants in the control group were matched for age, sex, ethnicity, and BMI (Table 1). Seventeen of the cannabis smokers (56%) also smoked tobacco, whereas only two participants in the control group were tobacco smokers. Systolic and diastolic blood pressures were significantly higher in cannabis users when compared with control participants (systolic blood pressure, 128 ± 13 vs. 120 ± 12 mm Hg, P = 0.01; diastolic blood pressure, 76 ± 10 vs. 71 ± 8 mm Hg, P = 0.01).
Table 1.
Demographics, cannabis use, energy intake, and dietary quality in chronic cannabis users and control nonusers
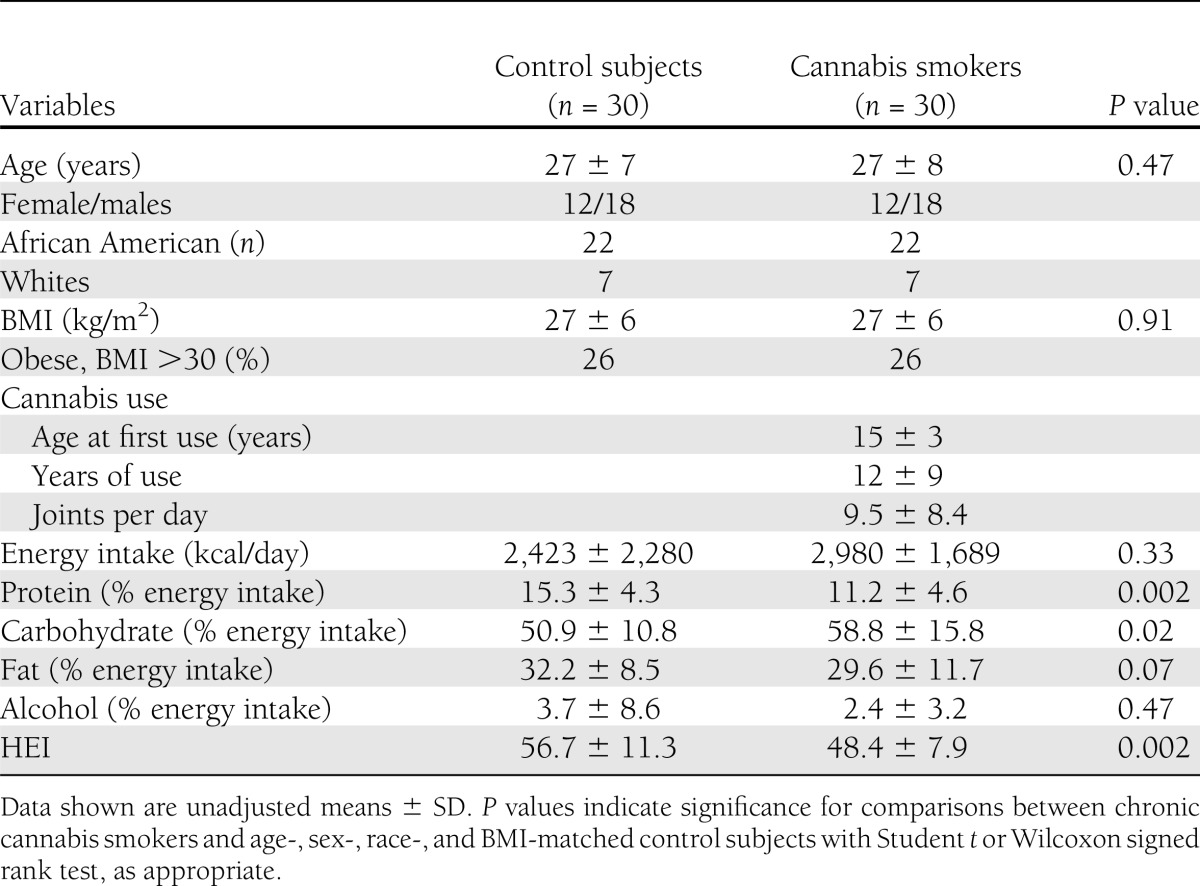
Total daily energy intake, assessed by 24-h dietary recall, was not significantly different between the groups. However, carbohydrate intake and percent calories from carbohydrates were significantly higher in cannabis smokers (Table 1). Diet quality was measured using the HEI-2005. As seen in Table 1, the quality of diet consumed by cannabis smokers was lower than the control group, reflecting differing food choices. Despite differences in total diet quality, the percent total daily calories from fat and alcohol were not significantly different between the groups. In both groups, women consumed less alcohol (% energy intake) than men (control subjects: 1.7 ± 2 vs. 5 ± 11; cannabis: 1.9 ± 4 vs. 2.7 ± 3).
Cannabis use, adiposity, and body fat distribution
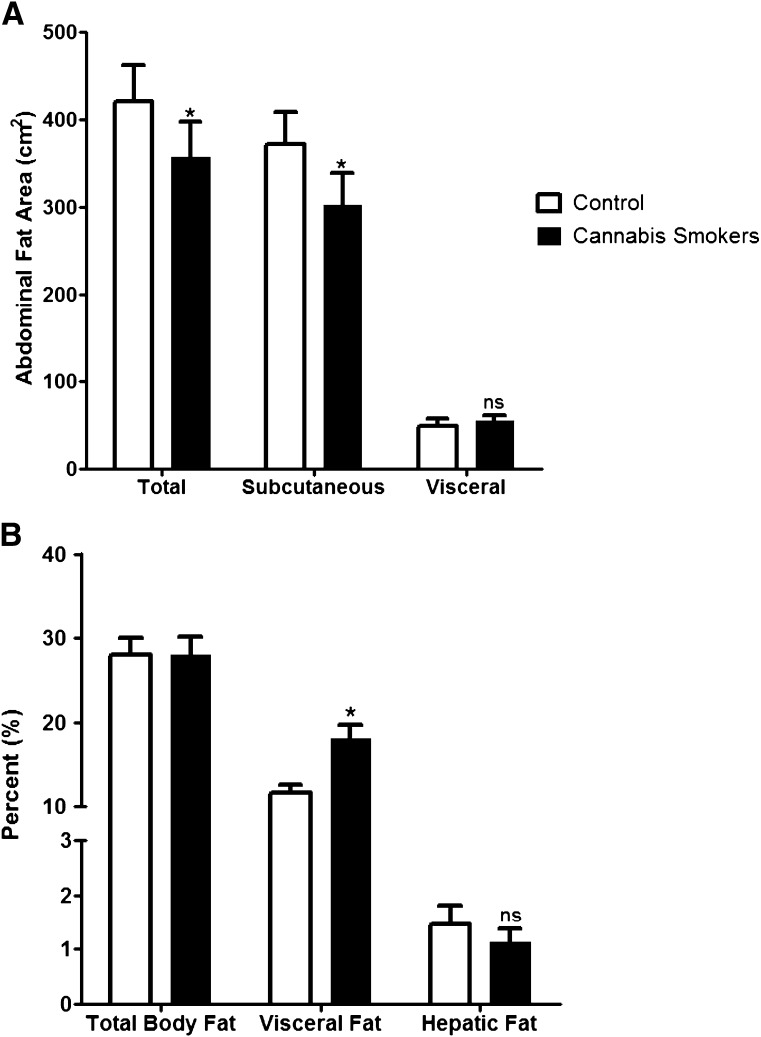
In addition to being age- and BMI-matched, total body fat (TBF) content (regional percent fat) was similar between the groups (28 ± 12 vs. 28 ± 11%; P = 0.75) (Fig. 1). Relative distribution of abdominal fat was evaluated by magnetic resonance imaging. While cannabis smokers had a lower total (P = 0.003) and subcutaneous (P = 0.008) fat in the abdomen, the percent of visceral fat area (visceral fat area/total abdominal fat area) was higher (18 ± 9 vs. 12 ± 5%; P = 0.004) when compared with the control group (Fig. 1). However, the percent of visceral fat area was unrelated to age or frequency or duration of cannabis use. Contrary to our hypothesis, hepatic fat content as assessed by MRS was not different between the groups (1.1 ± 1.3 vs. 1.5 ± 1.9%; P = 0.59) (Fig. 1). In the combined cohort, age (age: r = 0.26; P = 0.05) and percent TBF (r = 0.35; P = 0.007) was associated with hepatic fat content. Similar to percent visceral fat, hepatic fat content was unrelated to age or frequency or duration of cannabis use.
Figure 1.
Total adiposity and regional fat distribution in chronic cannabis smokers and control subjects. A: Abdominal total fat, subcutaneous fat, and visceral fat content. B: TBF (regional percent), visceral fat (percent of total abdominal fat), and hepatic fat content. Values shown are means ± SE. *P < 0.05, paired t test; ns, not significant.
Chronic cannabis use, metabolic parameters, and insulin sensitivity
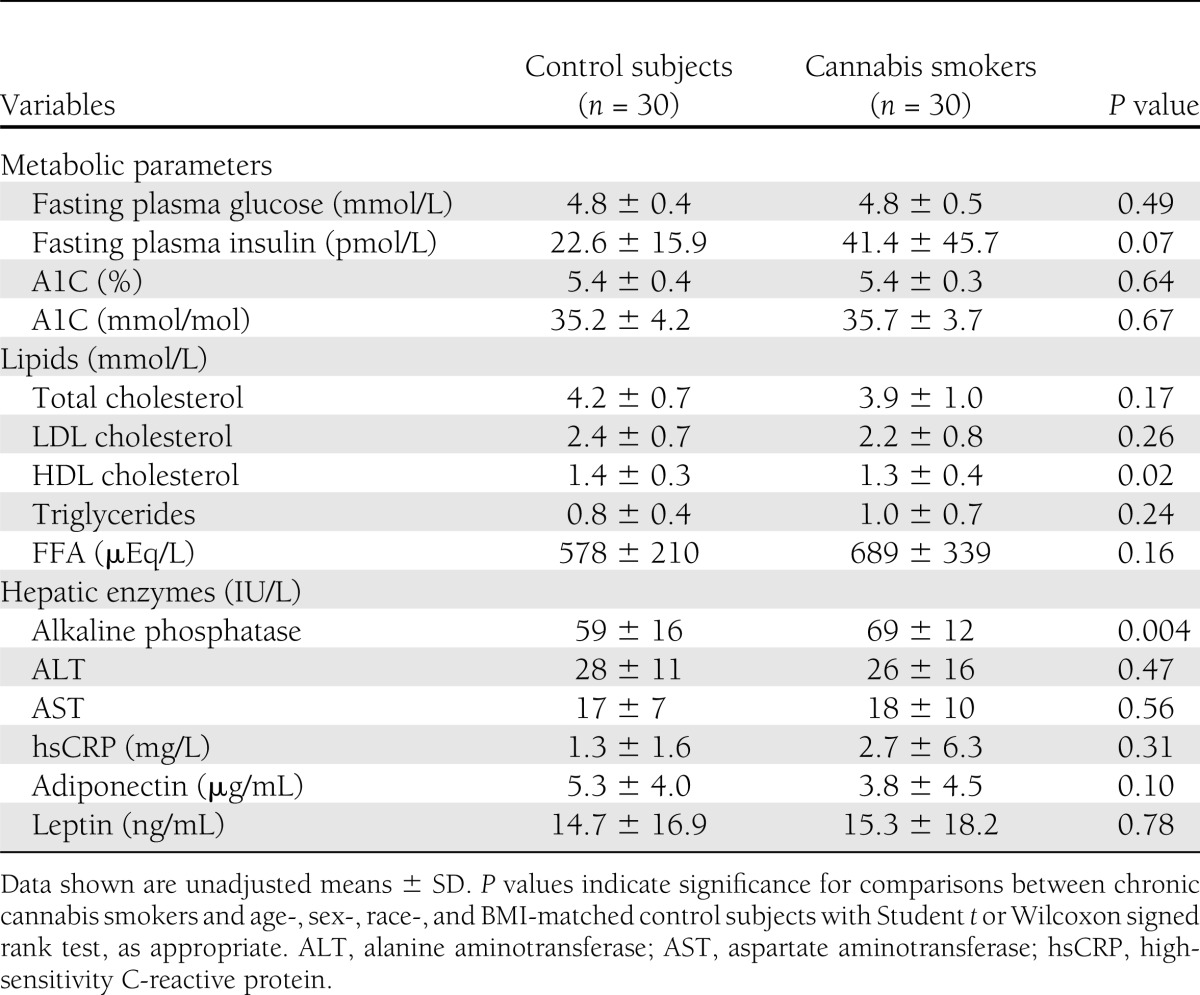
Fasting plasma glucose and insulin as well as a measure of glycemic control, A1C, were comparable between the groups (Table 2). Fasting levels of HDL cholesterol (P = 0.02) were lower in cannabis smokers (women: 49 ± 12 vs. 61 ± 12 mg/dL; men: 49 ± 12 vs. 51 ± 12 mg/dL). However, LDL cholesterol, triglycerides, and FFA levels were comparable between the groups (Table 2). Likewise, circulating levels of high-sensitivity C-reactive protein (cannabis: women, 5.6 ± 9.3 and men, 0.8 ± 0.7 mg/L; control subjects: women, 1.5 ± 1.9, men, 1.2 ± 1.4 mg/L), leptin (cannabis: women, 29.5 ± 20.0 and men, 5.9 ± 8.4 ng/mL; control subjects: women, 28.2 ± 18.4 and men, 5.1 ± 5.4 ng/mL), and adiponectin were similar (Table 2). Although, levels of liver transaminases, alanine aminotransferase, and aspartate aminotransferase were not different, plasma levels of alkaline phosphatase were significantly higher in cannabis smokers (Table 2).
Table 2.
Metabolic parameters in chronic cannabis users and control nonusers
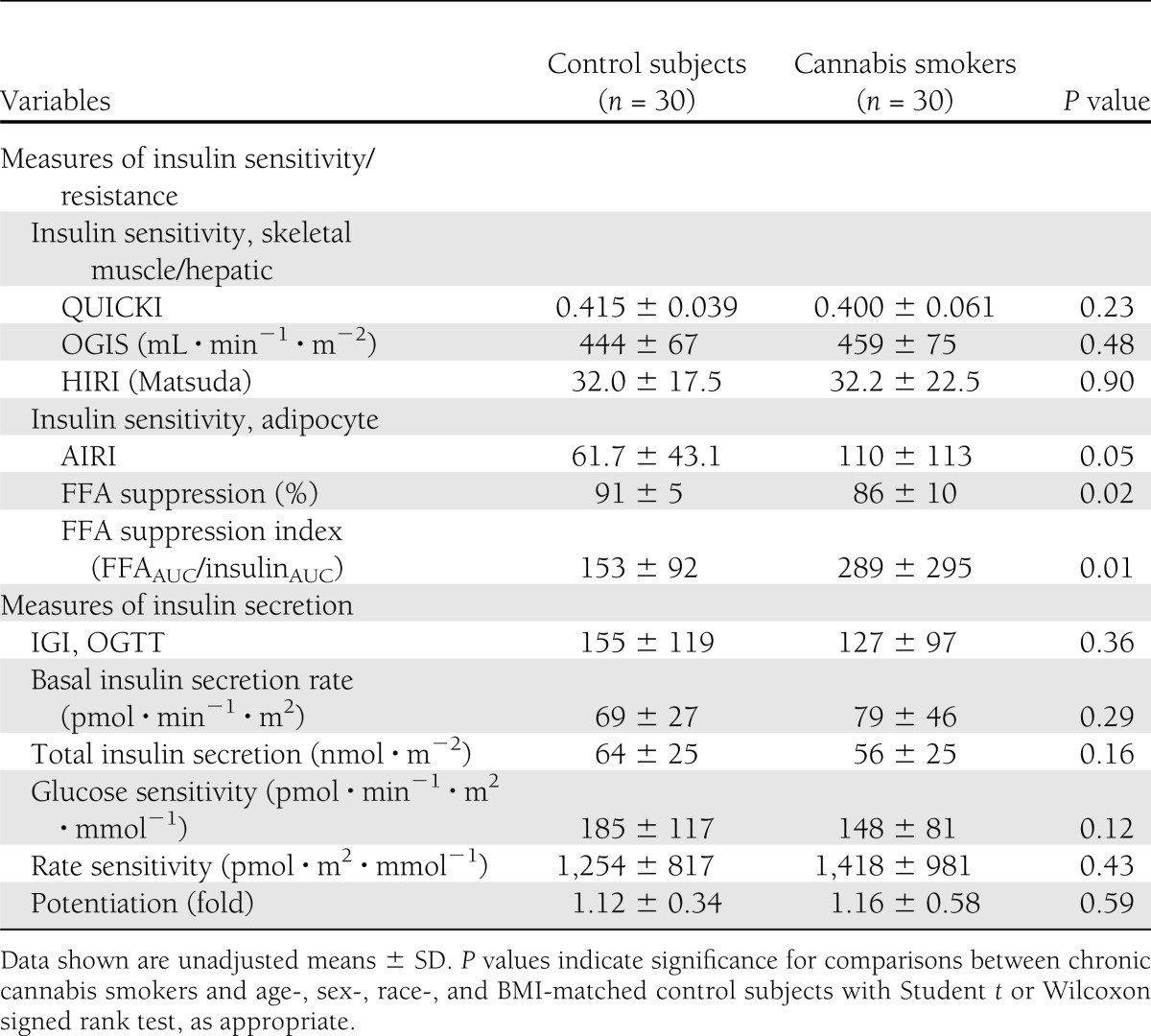
Measures of hepatic insulin resistance, QUICKI, HIRI, and OGIS, a measure of peripheral glucose disposal, were not significantly different between the groups (Table 3). However, indices of adipose tissue insulin resistance, AIRI, and FFA suppression index were significantly higher in cannabis smokers (Table 3).
Table 3.
Indices of insulin sensitivity/resistance, insulin secretion, and β-cell function in chronic cannabis users and control nonusers
Chronic cannabis use, insulin secretion, and pancreatic β-cell function
Basal and postglucose load absolute insulin secretory rates were comparable between the groups. In order to assess insulin secretion in response to a dynamic change in plasma glucose levels, we measured rate sensitivity and IGI. IGI, the ratio of the rise of the insulin and glucose concentrations over basal level at 30 min (ΔI/ΔG), and rate sensitivity were not different between the groups (Table 3). Insulin secretion and its relationship to plasma glucose concentrations were evaluated by model-derived β-cell glucose sensitivity parameter. Other measures of β-cell function, β-cell glucose sensitivity, and potentiation factor in chronic cannabis smokers were also similar to control subjects.
Incretin secretion in chronic cannabis smokers
Incretins play an important role in regulating appetite and pancreatic β-cell function. To examine if chronic cannabis smoking affects incretin levels, we measured fasting and post-OGTT incretin concentrations. Fasting levels of acyl-ghrelin (10.6 ± 2 vs. 13.2 ± 8.2 pg/mL; P = 0.29), desacyl ghrelin (282 ± 152 vs. 279 ± 200 pg/mL; P = 0.64), PYY (68.2 ± 22.4 vs. 77.9 ± 46.2 pg/mL; P = 0.44), and active GLP-1 (4.5 ± 3.8 vs. 6.1 ± 7.2 pmol/L; P = 0.52) were not different between the groups. Also, post-OGTT concentrations of ghrelin and active GLP-1 were similar between the groups (data not shown).
CONCLUSIONS
In the current study, we demonstrated that chronic cannabis smokers had relative visceral adiposity and adipose tissue insulin resistance but not hepatic steatosis, glucose insulin insensitivity, impaired pancreatic β-cell function, glucose intolerance, or dyslipidemia compared with age-, sex-, ethnicity-, and BMI-matched control individuals. Our study results suggest that chronic, daily cannabis use may have differential tissue-specific effects on insulin sensitivity, but these effects appear to have minimal impact on glucose or lipid metabolism.
Marijuana use and clinical characteristics of study participants
Cannabis is the most frequently used illicit drug, especially in younger individuals, and accounts for the main reason for admission into substance abuse treatments (12). Moreover, during the past few years there have been further increases in the prevalence of marijuana use in the U.S. (12). Consequently, understanding the chronic metabolic effects of marijuana smoking are important, a topic that has not been adequately studied. We designed this study to metabolically phenotype healthy chronic cannabis users. Marijuana users in the general population are more likely to be younger (<30 years old), be male, have an average age at first use of ∼18 years, and to frequently use other illicit drugs (12). In this study, we excluded individuals who currently abused other illicit drugs other than cannabis and with history of alcohol abuse or dependency. In 2010, an ∼6.9 million current marijuana users used the drug on ≥20 days in the past month (12). However, as noted in the Coronary Artery Risk Development in Young Adults (CARDIA) Study (25) and Third National Health and Nutrition Examination Survey (NHANES III) participants (26), only 1–3% of current marijuana smokers are heavy users. In our cohort of daily cannabis smokers, the dose and frequency of marijuana use (average ∼10 joints/day) and chronicity (∼12 years) was substantial.
Marijuana use is associated with higher daily caloric intake. In the NHANES III and CARDIA study, heavy cannabis users had ∼20% higher calorie intake than nonusers (25,26). The increase in calories was from higher intake of all macronutrients. Specifically, the frequency and amount of consumption of soda, cheese, salty snacks, pork, and alcohol was higher in cannabis users. Consistent with other studies, the quality of diets consumed by cannabis users was poor (27). Furthermore, the percent of daily calories derived from carbohydrates relatively rich in simple sugars was significantly higher in marijuana smokers. These findings are consistent with human and animal studies demonstrating that cannabinoids stimulate food intake, specifically highly palatable sweet-tasting foods (28). Cannabis smokers in our study exhibited characteristics typically observed in marijuana smokers in the general population. In particular, the degree and duration of cannabis exposure in our study participants provided us the opportunity to clearly assess any significant metabolic effects of chronic and heavy marijuana use in healthy individuals.
Effects of marijuana on adiposity and abdominal fat distribution
Δ9-THC, the lipophilic active ingredient of cannabis, is known to accumulate in adipose tissue of heavy marijuana users (29). This may explain adipose tissue–specific effects of chronic marijuana use. In in vitro studies, Δ9-THC has been shown to increase adipocyte hypertrophy and lipogenesis (30). Based on extant literature, mainly from in vitro and animal studies (2,3), we hypothesized that chronic cannabis users may have higher amounts of hepatic fat and abdominal visceral fat. Using MRS, we did not observe any significant differences in hepatic fat content between chronic cannabis users and age-, sex-, ethnicity-, and BMI-matched nonusers. In one recent epidemiological study of several hundred patients with hepatitis C infection, daily cannabis use was found to be a predictor of hepatic steatosis (by microscopy) (11). However, there was a higher prevalence of hepatic steatosis in nonmarijuana users with HCV infection, suggesting that marijuana use may exacerbate the pathological effects of HCV infection in the liver. Alkaline phosphatase, but not hepatic transaminase, levels were higher in cannabis users in our study. This is consistent with a prior report demonstrating elevated hepatic enzymes and hepatomegaly in cannabis smokers (31). But for this solitary study, there are no other human studies that help interpret or refute the likelihood of a false-positive finding of higher levels of alkaline phosphatase.
In animal studies, chronic CB1R stimulation by endocannabinoids favors adiposity independent of its effects on appetite stimulation, as deduced from the opposite effects of chronic CB1R blockade. This contrasts with the effects of chronic marijuana use as reported in NHANES III (27), the National Epidemiologic Survey on Alcohol and Related Conditions, the National Comorbidity Survey–Replication (32), and the CARDIA (25) studies, which show that despite an increase in caloric intake, prevalence of obesity is lower in cannabis users as compared with nonusers. Moreover, in NHANES III, higher frequency of marijuana use was significantly associated with lower BMI (27). This negative association of cannabis use and BMI remained even after adjustment for potential confounders such as age, sex, education, caloric intake, tobacco smoking, and alcohol use.
In our study, nonmarijuana users had comparable amounts of percent TBF, but the total abdominal fat content (subcutaneous and visceral combined) was significantly lower in cannabis smokers. However, when we examined the relative amounts of abdominal fat distribution, cannabis smokers had significantly higher percentage of visceral fat. This finding is consistent with the observation that marijuana use is associated with larger waist circumference (25). In obese individuals, endocannabinoid levels are higher in abdominal (33–36), but paradoxically lower in subcutaneous adipose tissue (37,38). These findings have led to the suggestion that such depot-specific upregulation of endocannabinoid system (ECS) may favor partition of lipids toward visceral and away from subcutaneous fat depots during states of caloric excess (39). Interestingly, Δ9-THC–induced decrease in AMP-activated protein kinase, an enzyme that activates fatty acid oxidation, is greater in visceral adipocytes (40), suggesting a depot-specific effect. Although not known, such depot-specific actions of phytocannabinoids may explain the effects of chronic cannabis smoking on regional fat distribution. In addition, non–Δ9-THC phytocannabinoids (e.g., cannabidiol [CBD]) may also play a role in adipogenesis (41). However, the ratio of Δ9-THC versus CBD exceeded 10:1 in the vast majority of marijuana samples seized in Maryland (M. A. ElSohly, personal communication). This makes it unlikely that non–Δ9-THC cannabinoids such as CBD contributed to the relative visceral adiposity observed in our study. These novel and intriguing findings from our study should be considered hypothesis-generating that need further confirmation in larger studies.
Effects of marijuana use on insulin action and secretion
Daily smoking of marijuana for 7 days or acute intravenous administration of Δ9-THC induces glucose intolerance in men (8,9). However, the effects of chronic marijuana smoking on glucose disposal, insulin action, and β-cell function have not been previously investigated. In our study, chronic cannabis impaired adipose tissue insulin sensitivity but not insulin sensitivity parameters related to glucose metabolism. These tissue-specific effects of marijuana may be due to: 1) higher levels of Δ9-THC and prolonged retention in adipose tissue (29); 2) higher sensitivity to antilipolytic effects of insulin in adipose tissue compared with its action in the liver (suppression of glucose production) or skeletal muscle (stimulation of glucose disposal); and 3) varying expression of CB1R in insulin-sensitive tissues (e.g., expression of CB1Rs in liver is minimal in healthy individuals) (42).
Pancreatic CB1R activation leads to β-cell death and impairs insulin secretion in mice (4). Whether chronic marijuana smoking affects insulin secretion in humans is not known. Using C-peptide deconvolution and OGTT modeling, total insulin secretion, β-cell glucose sensitivity, rate sensitivity, and potentiation of insulin secretion were evaluated. Chronic cannabis smoking does not appear to impair β-cell glucose sensitivity, rate sensitivity, or insulin secretion. Thus, chronic marijuana smokers manifest normal glucose tolerance due to intact peripheral insulin sensitivity and β-cell function. The lack of adverse effects of chronic cannabis use on glucose tolerance is consistent with the lower prevalence of type 2 diabetes in heavy marijuana smokers (26). Likewise, heavy marijuana use did not impair fasting glucose levels in the CARDIA study (25).
Marijuana use, blood pressure, and dyslipidemia
Chronic marijuana users had higher blood pressure but near-normal lipid parameters compared with nonusers. Similar findings have also been reported from the NHANES III and CARDIA study (25,26). One potential confounder in interpreting these findings is the frequent association of tobacco smoking in chronic cannabis smokers in our and other's studies (12,25).
Marijuana use and appetite-modifying incretins
Appetite stimulating actions of cannabis are well established, but the effects of chronic cannabis use on appetite hormones are unknown. In a recent study, administration of medicinal cannabis acutely increases plasma ghrelin and leptin and decreases PYY levels in individuals with neuropathic pain (43). Although we did not assess appetite ratings, fasting and post-OGTT levels of ghrelin, GLP-1, and PYY were unaffected by marijuana smoking. It is probable that the duration of cannabis exposure (acute vs. chronic) may differentially affect levels of circulating appetite hormones. Further studies are needed to confirm these findings.
Metabolic effects of phytocannabinoids versus endocannabinoids
Phytocannabinoids are plant-derived natural products that are chemically similar to cannabinoids and interact with CB receptors. The two major cannabinoids from cannabis sativa are Δ9-THC and CBD. Δ9-THC is a CB1R and CB2R partial agonist, and CBD produces a broad range of effects not mediated by either CB1R or CB2R, although in some systems it may behave as a CB2R inverse agonist (44). However, when CB receptor expression is low, Δ9-THC blocks CB receptor activation by other cannabinoids (44). Chronic cannabis use downregulates CB1Rs (45) and may induce tolerance to the effects of Δ9-THC. This is the most likely explanation of the discrepant effects of cannabis on glucose metabolism in short-term studies and after chronic exposure. Although the possibility of CB1R desensitization exists, definitive conclusions cannot be made due to lack of data regarding cannabis dose over time (stable vs. scaling up), especially at the time of metabolic assessment. The ability of Δ9-THC to block or activate CB receptors, differential downregulation of CB1Rs, role of non-CB1R activation by phytocannabinoids, varying tissue-specific expression of CB receptors, and the development of tolerance to some effects of Δ9-THC may explain the differential effects of ECS upregulation in rodents and chronic cannabis use in humans.
Limitations
Our study has several limitations aside from the small cohort size and lack of multiplicity corrections for exploratory outcomes. First, the accuracy of self-reported substance use is frequently questioned due to substantial underreporting. However, in our study, cannabis use was elicited by experienced investigators and underreporting minimized through assurances of confidentiality, lack of adverse consequences, appropriate recall cues, and other confirmatory biological markers for confirmation (46). Even if underreported, cannabis use was significant in our study. Second, although the effect of tobacco smoking on insulin sensitivity is equivocal (47,48), the high prevalence of concurrent tobacco use in cannabis users is inevitably a major confounder. Nonetheless, the metabolic parameters were not different between tobacco users and nonusers in cannabis users. Third, consumption of a diet rich in carbohydrates with high glycemic index may have had independent effects on adipose tissue insulin sensitivity and visceral adiposity. However, carbohydrate consumption was assessed by dietary history, which is highly variable, especially in our cannabis cohort. Fourth, we used surrogate measures of insulin sensitivity and secretion. Although these indices and β-cell function modeling have been extensively validated and used, the findings from our exploratory study need to be confirmed using gold-standard techniques such as euglycemic hyperinsulinemic and hyperglycemic clamp techniques. Fifth, given the high proportion of African Americans in our cohort, our results may have also been affected by the relative resistance of African Americans to hepatic steatosis (49). Finally, confounding effects of varying composition of cannabis preparations and the relative amounts and activities of non-THC cannabinoids (e.g., CBD, cannabigerol, and tetrahydrocannabivarin) and even noncannabinoids such as terpenoids on glucose metabolism is an unavoidable limitation.
In healthy young individuals, chronic cannabis smoking was associated with visceral adiposity and adipose tissue insulin resistance but not with hepatic steatosis, insulin insensitivity, impaired pancreatic β-cell function, or glucose intolerance.
Acknowledgments
This work was supported by the Intramural Research Programs of National Institute of Diabetes and Digestive and Kidney Diseases, National Institute on Drug Abuse, and National Institute on Alcohol Abuse and Alcoholism, National Institutes of Health.
No potential conflicts of interest relevant to this article were reported.
R.M. participated in the conduct of the study, data analysis, and writing of the manuscript and approved the final manuscript. S.S., R.O., A.M.G., M.W., A.C., and G.H. participated in the conduct of the study and collection of data and approved the final manuscript. A.M. participated in the data analysis and approved the final manuscript. K.Y.C. participated in the data analysis and writing of the manuscript and approved the final manuscript. N.D.V. and G.K. participated in the design of the study and writing of the manuscript and approved the final manuscript. M.A.H. participated in the design and conduct of the study, collection of data, and writing of the manuscript and approved the final manuscript. M.C.S. participated in the design and conduct of the study, collection of data, and writing of the manuscript and approved the final manuscript. M.C.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at The Endocrine Society's 94th Annual Meeting & Expo, Houston, Texas, 23–26 June 2012.
Footnotes
Clinical trial reg. no. NCT00428987, clinicaltrials.gov.
References
- 1.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 2006;58:389–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006;27:73–100 [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Zhou L, Xiong K, et al. Hepatic cannabinoid receptor-1 mediates diet-induced insulin resistance via inhibition of insulin signaling and clearance in mice. Gastroenterology 2012;142:1218–1228e1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim W, Lao Q, Shin YK, et al. Cannabinoids induce pancreatic β-cell death by directly inhibiting insulin receptor activation. Sci Signal 2012;5:ra23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osei-Hyiaman D, DePetrillo M, Pacher P, et al. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest 2005;115:1298–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Després JP, Golay A, Sjöström L, Rimonabant in Obesity-Lipids Study Group Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 2005;353:2121–2134 [DOI] [PubMed] [Google Scholar]
- 7.Triay J, Mundi M, Klein S, et al. Does rimonabant independently affect free fatty acid and glucose metabolism? J Clin Endocrinol Metab 2012;97:819–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hollister LE, Reaven GM. Delta-9-tetrahydrocannabinol and glucose tolerance. Clin Pharmacol Ther 1974;16:297–302 [DOI] [PubMed] [Google Scholar]
- 9.Podolsky S, Pattavina G, Amaral M. Effect of marijuana on the glucose-tolerance test. Ann NY Acad Sci 1971;191:54–60 [Google Scholar]
- 10.Foltin RW, Fischman MW, Byrne MF. Effects of smoked marijuana on food intake and body weight of humans living in a residential laboratory. Appetite 1988;11:1–14 [DOI] [PubMed] [Google Scholar]
- 11.Hézode C, Zafrani ES, Roudot-Thoraval F, et al. Daily cannabis use: a novel risk factor of steatosis severity in patients with chronic hepatitis C. Gastroenterology 2008;134:432–439 [DOI] [PubMed] [Google Scholar]
- 12.Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings [Internet]. Rockville, MD, Substance Abuse and Mental Health Services Administration. Available from http://www.samhsa.gov/data/nsduh/2k10nsduh/2k10results.htm Accessed 12 February 2013
- 13.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol 2001;154:1089–1099 [DOI] [PubMed] [Google Scholar]
- 14.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1896–1901 [DOI] [PubMed] [Google Scholar]
- 15.van der Meer RW, Lamb HJ, Smit JW, de Roos A. MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology 2012;264:21–37 [DOI] [PubMed] [Google Scholar]
- 16.Ouwerkerk R, Pettigrew RI, Gharib AM. Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology 2012;265:565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab 2008;294:E15–E26 [DOI] [PubMed] [Google Scholar]
- 18.Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care 2001;24:539–548 [DOI] [PubMed] [Google Scholar]
- 19.Abdul-Ghani MA, Matsuda M, Balas B, DeFronzo RA. Muscle and liver insulin resistance indexes derived from the oral glucose tolerance test. Diabetes Care 2007;30:89–94 [DOI] [PubMed] [Google Scholar]
- 20.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 21.Abdul-Ghani MA, Molina-Carrion M, Jani R, Jenkinson C, Defronzo RA. Adipocytes in subjects with impaired fasting glucose and impaired glucose tolerance are resistant to the anti-lipolytic effect of insulin. Acta Diabetol 2008;45:147–150 [DOI] [PubMed] [Google Scholar]
- 22.Tschritter O, Fritsche A, Thamer C, et al. Plasma adiponectin concentrations predict insulin sensitivity of both glucose and lipid metabolism. Diabetes 2003;52:239–243 [DOI] [PubMed] [Google Scholar]
- 23.Mari A, Schmitz O, Gastaldelli A, Oestergaard T, Nyholm B, Ferrannini E. Meal and oral glucose tests for assessment of beta-cell function: modeling analysis in normal subjects. Am J Physiol Endocrinol Metab 2002;283:E1159–E1166 [DOI] [PubMed] [Google Scholar]
- 24.Seltzer HS, Allen EW, Herron AL, Jr, Brennan MT. Insulin secretion in response to glycemic stimulus: relation of delayed initial release to carbohydrate intolerance in mild diabetes mellitus. J Clin Invest 1967;46:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodondi N, Pletcher MJ, Liu K, Hulley SB, Sidney S, Coronary Artery Risk Development in Young Adults (CARDIA) Study Marijuana use, diet, body mass index, and cardiovascular risk factors (from the CARDIA study). Am J Cardiol 2006;98:478–484 [DOI] [PubMed] [Google Scholar]
- 26.Rajavashisth TB, Shaheen M, Norris KC, et al. Decreased prevalence of diabetes in marijuana users: cross-sectional data from the National Health and Nutrition Examination Survey (NHANES) III. BMJ Open 2012;2:e000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smit E, Crespo CJ. Dietary intake and nutritional status of US adult marijuana users: results from the Third National Health and Nutrition Examination Survey. Public Health Nutr 2001;4:781–786 [DOI] [PubMed] [Google Scholar]
- 28.Abel EL. Effects of marihuana on the solution of anagrams, memory and appetite. Nature 1971;231:260–261 [DOI] [PubMed] [Google Scholar]
- 29.Johansson E, Norén K, Sjövall J, Halldin MM. Determination of delta 1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed Chromatogr 1989;3:35–38 [DOI] [PubMed] [Google Scholar]
- 30.Wong A, Gunasekaran N, Hancock DP, et al. The major plant-derived cannabinoid Δ(9)-tetrahydrocannabinol promotes hypertrophy and macrophage infiltration in adipose tissue. Horm Metab Res 2012;44:105–113 [DOI] [PubMed] [Google Scholar]
- 31.Borini P, Guimaraes RC, Borini SB. Possible hepatotoxicity of chronic marijuana usage. Sao Paulo Med J 2004;122:110–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Strat Y, Le Foll B. Obesity and cannabis use: results from 2 representative national surveys. Am J Epidemiol 2011;174:929–933 [DOI] [PubMed] [Google Scholar]
- 33.Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 2006;91:3171–3180 [DOI] [PubMed] [Google Scholar]
- 34.Blüher M, Engeli S, Klöting N, et al. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 2006;55:3053–3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Côté M, Matias I, Lemieux I, et al. Circulating endocannabinoid levels, abdominal adiposity and related cardiometabolic risk factors in obese men. Int J Obes (Lond) 2007;31:692–699 [DOI] [PubMed] [Google Scholar]
- 36.Di Marzo V, Côté M, Matias I, et al. Changes in plasma endocannabinoid levels in viscerally obese men following a 1 year lifestyle modification programme and waist circumference reduction: associations with changes in metabolic risk factors. Diabetologia 2009;52:213–217 [DOI] [PubMed] [Google Scholar]
- 37.Annuzzi G, Piscitelli F, Di Marino L, et al. Differential alterations of the concentrations of endocannabinoids and related lipids in the subcutaneous adipose tissue of obese diabetic patients. Lipids Health Dis 2010;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bennetzen MF, Wellner N, Ahmed SS, et al. Investigations of the human endocannabinoid system in two subcutaneous adipose tissue depots in lean subjects and in obese subjects before and after weight loss. Int J Obes (Lond) 2011;35:1377–1384 [DOI] [PubMed] [Google Scholar]
- 39.Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia 2008;51:1356–1367 [DOI] [PubMed] [Google Scholar]
- 40.Kola B, Hubina E, Tucci SA, et al. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem 2005;280:25196–25201 [DOI] [PubMed] [Google Scholar]
- 41.De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011;163:1479–1494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teixeira-Clerc F, Julien B, Grenard P, et al. CB1 cannabinoid receptor antagonism: a new strategy for the treatment of liver fibrosis. Nat Med 2006;12:671–676 [DOI] [PubMed] [Google Scholar]
- 43.Riggs PK, Vaida F, Rossi SS, et al. A pilot study of the effects of cannabis on appetite hormones in HIV-infected adult men. Brain Res 2012;1431:46–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 2008;153:199–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hirvonen J, Goodwin RS, Li CT, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012;17:642–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Donovan DM, Bigelow GE, Brigham GS, et al. Primary outcome indices in illicit drug dependence treatment research: systematic approach to selection and measurement of drug use end-points in clinical trials. Addiction 2012;107:694–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henkin L, Zaccaro D, Haffner S, et al. Cigarette smoking, environmental tobacco smoke exposure and insulin sensitivity: the Insulin Resistance Atherosclerosis Study. Ann Epidemiol 1999;9:290–296 [DOI] [PubMed] [Google Scholar]
- 48.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance—a potential link with the insulin resistance syndrome. J Intern Med 1993;233:327–332 [DOI] [PubMed] [Google Scholar]
- 49.Guerrero R, Vega GL, Grundy SM, Browning JD. Ethnic differences in hepatic steatosis: an insulin resistance paradox? Hepatology 2009;49:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]