Figure 1.
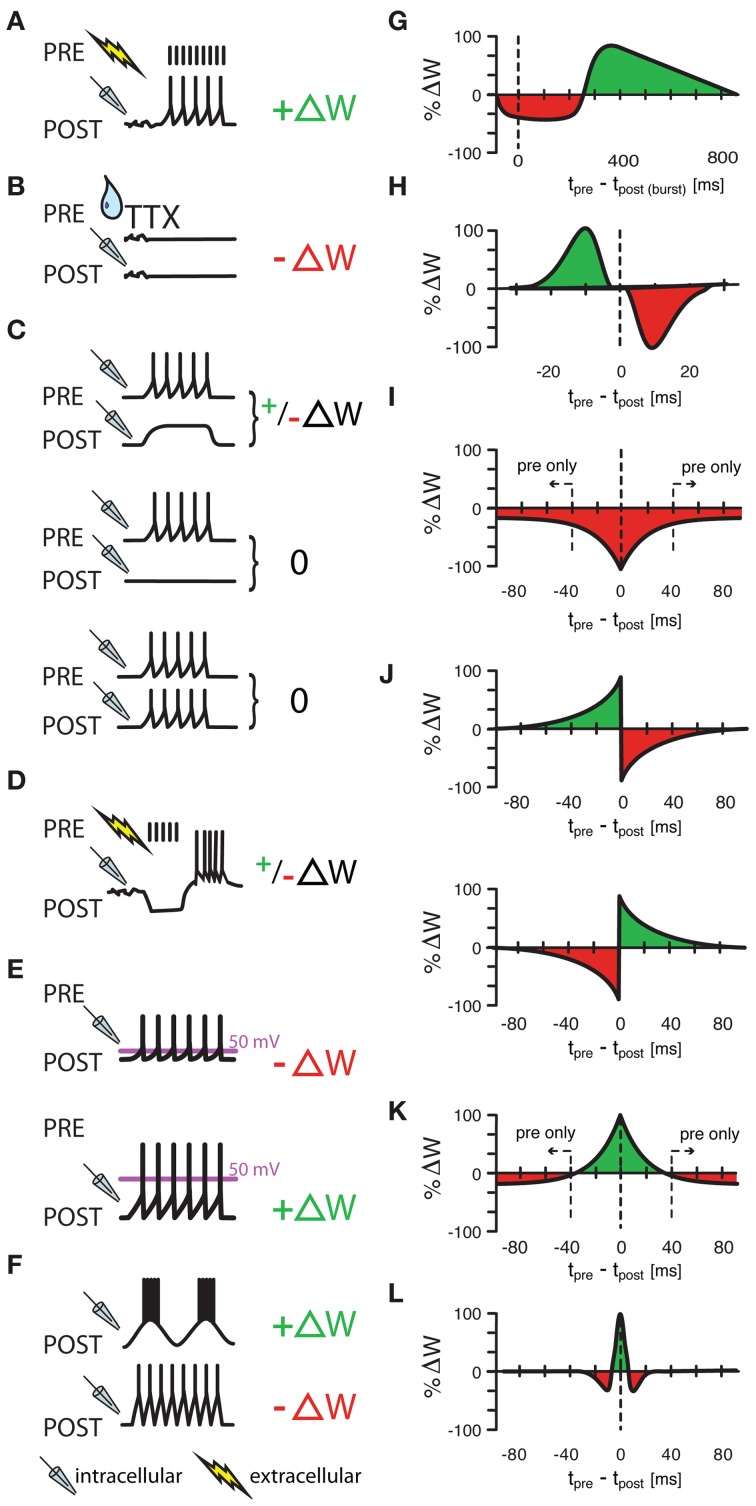
Various protocols of ISP induction. (A) Homeostatic plasticity induced by Hartmann et al., (2008) in >4 week old mouse CA1 hippocampal slices. A one second long extracellular stimulus of 100 Hz, delivered in the presence of glutamatergic and GABAB receptor blockers provoked a strengthening of GABAergic synapses through increased presynaptic GABA concentrations. (B) A similarly homeostatic response was induced by Kilman et al., (2002) in cultures of P3–P5 rat visual cells. Here, 2 day long silencing of the culture with TTX led to decreased amplitude of inhibitory post synaptic potentials (and loss of synapses) that was mediated by a decrease in GABAA receptors. (C) Congruent with (A,B), Maffei et al., (2006) showed that a postsynaptic depolarization in the presence of presynaptic bursts (20 bursts of ten presynaptic action potentials at 50 Hz) strengthens synapses in slices of P21 rat visual cortex with normal activity, but weakens synapses in previously monocularly deprived animals [and thus slices with consequently lower baseline activity and presumably already potentiated inhibitory synapses, (C), upper panel]. Presynaptic bursts, coupled with postsynaptic silence or firing did not induce any change at all [(C), middle and lower panel, respectively]. (D–F) Other protocols had “non-homeostatic” effects: Aizenman et al., (1998) induced synaptic weight changes (with 10 spike bursts at 2 Hz) in inhibitory synapses in P11–P15 coronal slices of rat cerebellum that were dependent on the postsynaptic firing frequency of the inhibitory rebound burst (D) and Kurotani et al., (2008) could control ISP in slices of layer V primary visual cortex of P20–P30 rats by altering either the postsynaptic rest potential during intracellular (sole postsynaptic) stimulation (15 5s-bursts at 20 Hz) (E), or by modifying the frequency of postsynaptic sub-threshold membrane fluctuations (F). Additionally to these non-spike timing-dependent protocols, three experimental studies (G–I) have shown spike-timing dependence under certain conditions. Holmgren and Zilberter, (2001) successfully manipulated the amplitude of synaptic weight changes in P14—P16 somatosensory cortex slices of rat by pairing postsynaptic bursts (25–40 times 10 spikes at 50 Hz) with single presynaptic spikes at up to 800 ms after the onset of the burst (G). Conversely, Haas et al., (2006) found bidirectional plasticity windows (H) on timescales more reminiscent of the classical excitatory STDP window in P14—P21 rat slices of entorhinal cortex and Woodin et al., (2003) found monodirectional plasticity in rat hippocampus cultures and slices. Interestingly, temporally proximal spike pairs weakened synaptic efficacy (measured from rest) through local changes in chloride reversal but sole presynaptic events decreased the amplitude of synaptic conductance (I). (J–L) Other learning rules have been tested in models, but have not been observed in experiments. Luz and Shamir, (2012) used Hebbian and Anti-Hebbian variations of classical, asymmetric STDP windows (J), as well as a symmetric form of iSTDP (K) also used by Vogels et al., (2011) that lead to strengthened synapses for near coincident spike pairs, but to weakened synapses for sole presynaptic events. Gilson et al., (2012) used a similar, mexican-hat shaped learning rule to produce experimentally observable frequency response behaviors (L). ΔW stands for a change in synaptic weight. In panel (A–F) a drop symbolizes the use of TTX; a flash symbolizes the use of an extracellular, and a pipette the use of an intracellular electrode. All values in (G–L) have been normalized to the maximum value of each data set.