Abstract
OBJECTIVE:
Hypoalbuminemia is a common clinical deficiency in burn patients and is associated with complications related to increased extravascular fluid, including edema, abnormal healing, and susceptibility to sepsis. Some prognostic scales do not include biochemical parameters, whereas others consider them together with comorbidities. The purpose of this study was to determine whether serum albumin can predict mortality in burn patients.
METHODS:
We studied burn patients ≥16 years of age who had complete clinical documentation, including the Abbreviated Burn Severity Index, serum albumin, globulin, and lipids. Sensitivity and specificity analyses were performed to determine the cut-off level of albumin that predicts mortality.
RESULTS:
In our analysis of 486 patients, we found that mortality was higher for burns caused by flame (p = 0.000), full-thickness burns (p = 0.004), inhalation injuries (p = 0.000), burns affecting >30% of the body surface area (p = 0.001), and burns associated with infection (p = 0.008). Protein and lipid levels were lower in the patients who died (p<0.05). Albumin levels showed the highest sensitivity and specificity (84% and 83%, respectively), and the area under the receiver-operating characteristic curve (0.869) had a cut-off of 1.95 g/dL for mortality.
CONCLUSION:
Patients with albumin levels <2 g/dL had a mortality risk of >80%, with 84% sensitivity and 83% specificity. At admission, the albumin level could be used as a sensitive and specific marker of burn severity and an indicator of mortality.
Keywords: Burns, Level of Severity of Injury, Serum Albumin, Mortality
INTRODUCTION
From 2004 to 2008, the Mexican National Health Information System (SINAIS) reported 65,896 discharges of patients affected by burn injuries from national public institutions, 64,606 (98.04%) of which were attributed to morbidity and 1,290 (1.96%) to mortality. During the same period, SINAIS also reported a total of 3,795 discharges from public institutions in the state of Jalisco, Mexico, 3,736 of which (98.44%) were attributed to morbidity and 59 (1.6%) to mortality (1,2).
Globally, advances in treating burns have significantly changed the clinical course of a patient's recovery, increasing the chance of survival. However, the mortality rate remains high, and the probability of death can be predicted from clinical factors (3–5).
Evaluating the risk of death in burn patients is essential for their overall treatment and selecting and improving their future management regimens (4). The use of prognostic factors has been attempted in these patients, and several scales, such as the Abbreviated Burn Severity Index (ABSI), which includes variables such as sex, age, total burned body surface area (BSA), full-thickness injuries, and burns attributable to inhalation (6), have been implemented but do not include biochemical variables. The Acute Physiology and Chronic Health Evaluation (APACHE) II and APACHE III scales include serum albumin levels and comorbidities to improve predictive power (7).
Hypoalbuminemia is common in critically ill patients, particularly in burn patients (8). Even when burns cover <10% of the body surface, important metabolic changes occur. Burns produce hypermetabolic and hypercatabolic responses, which are related to the extent and depth of the injuries (9). Burns affecting >20% of the body surface cause a major loss of extracellular fluids, thereby inducing shock by increasing vascular permeability and reducing plasma albumin from the wound exudations. Hypoalbuminemia also causes complications related to increasing extravascular fluids, including edema, abnormalities in healing, and increased susceptibility to sepsis (10). However, it is unclear whether hypoalbuminemia can be used as a predictor of mortality. Therefore, we investigated whether hypoalbuminemia could predict mortality in burn patients.
MATERIALS AND METHODS
Design
We performed a retrospective cross-sectional study of burn patients admitted to the Burn Unit of the Specialties Hospital of the Western National Medical Center in Guadalajara, Mexico, from January 2009 to December 2011.
During this 36-month period, we included individuals older than 16 years of age who were diagnosed with burns within 24 hours after injury (regardless of the causative agent, location, or thickness of the burn) and who had complete clinical documentation. We excluded patients with insufficient information to analyze the appropriate variables and patients suffering end-stage kidney disease, nephrotic syndrome, chronic liver disease, or inflammatory bowel disease, or were undernourished. The variables examined were age, gender, causal agent, occupation, site of injury, burned BSA, full-thickness burns, and inhalation burns; laboratory test results, such as hemoglobin, hematocrit, total leukocytes, lymphocytes, neutrophils, platelet count, blood glucose, urea, creatinine, cholesterol, serum total proteins, albumin, and globulin at admission to the Burn Unit, were also examined. The calculation of the burn BSA was based on the “rule of nines”. This system is useful for rapidly assessing adults, and second- and third-degree burns were included and corrected according to the Lund–Browder scheme (11). Albumin levels were considered to be both a quantitative variable and an ordinal variable and were divided into three groups: >3 g/dL, 2–3 g/dL, and <2 g/dL. Inhalation injury was diagnosed by direct laryngoscopy and/or bronchoscopy based on positive findings of redness, mucosal edema, ash particles, and vascular injection in addition to evidence of burns to the face, enclosed spaces, and nasal vibrissae.
Full-thickness burns were defined as third-degree burns that involved the destruction of the entire thickness of the epidermis and dermis, including the appendices. These burns were stiff and whitish in appearance and presented with blood vessel coagulation and sensory nerve destruction; edema was present below the damaged tissue despite the scarring. We defined “expected mortality” as the probability of death according to the ABSI scale, and “observed mortality” as the percentage of deaths in the population.
Statistical analysis
The descriptive phase of the analysis included the presentation of data as raw proportions, means, and standard deviations. The Student's t-test was used to compare the quantitative variables, and a χ2 test was used for the qualitative variables. A univariate analysis was performed to determine the odds ratios (OR) and 95% confidence intervals (CIs), and an analysis of the sensitivity and specificity for the quantitative variables was conducted. The cut-off points at which the levels of serum albumin, globulin, total proteins, cholesterol, and triglycerides (TG) established a sensitivity and specificity of 80% were determined, and p<0.05 was considered significant. Microsoft Excel 2007 (Redmond, WA, USA) and SPSS for Windows (version 17; IBM, Armonk, NY, USA) were used for data processing and statistical analysis. The protocol was approved by the Local Committee on Health Research, code 2008-1301-77.
RESULTS
During the study period, 652 patients were admitted, 486 of whom met the inclusion criteria for this study. The remaining 166 patients were excluded because of previous treatment in another hospital or burn unit, associated morbidity, unavailability for follow-up because of transfer to another medical unit, and/or incomplete medical records. Most of the patients were male (373 cases, 76.7%), and 113 were female (23.3%). The patients ranged in age from 22 to 56 years (average age, 38.9±16.6 years), and 65.6% were married. The accident occurred in the workplace in 51.9% of cases and at home in 33.5%; the remainder (14.6%) occurred as a result of traffic accidents, sporting events, or during school hours. The characteristics, causes, extents, and depths of the lesions are described in Table 1. Most individuals had burns to ≤30% of their BSA (83.1%), and >30% of the BSA was affected in 16.9% of patients. The most common causative agent was flame (50.6%). The expected mortality according to the ABSI scale is described in Table 2. Mortality was observed in 35 patients (7.2%) during the study period. One patient with an ABSI score in the 4–5 range died, although the expected mortality was only 2%. Of the remaining 34 non-surviving patients, those with scores exceeding 8–9 had an expected mortality of up to 50% but an observed mortality of only 10.5%. In patients with scores >12, the expected mortality was >90%, and the observed mortality was 66.6%. The ABSI scores upon admission were 9.37±2.59 in the patients who died and 5.02±1.8 in those who survived.
Table 1.
Characteristics of the burn injuries in 486 patients.
| Causative agent | No. of patients | % |
| Flame | 246 | 50.6 |
| Electricity | 130 | 26.7 |
| Scalding | 84 | 17.3 |
| Others | 26 | 5.3 |
| <30% of body surface affected | 404 | 83.1 |
| >30% of body surface affected | 82 | 16.9 |
| Full-thickness | 156 | 32.1 |
| Injury caused by inhalation | 61 | 12.6 |
Table 2.
Expected and observed mortality according to the ABSI scale (n = 486).
| ABSI points | Affected n (%) | Observed mortality n (%) | Expected mortality % |
| 2–3 | 90 (18.6) | 0 | <1 |
| 4–5 | 215 (44.2) | 1 (0.46) | 2 |
| 6–7 | 115 (23.7) | 12 (10.4) | 10–20 |
| 8–9 | 38 (7.8) | 4 (10.5) | 30–50 |
| 10–11 | 16 (3.2) | 10 (62.5) | 60–80 |
| 12–13 | 12 (2.4) | 8 (66.6) | >90 |
ABSI, Abbreviated Burn Severity Index.
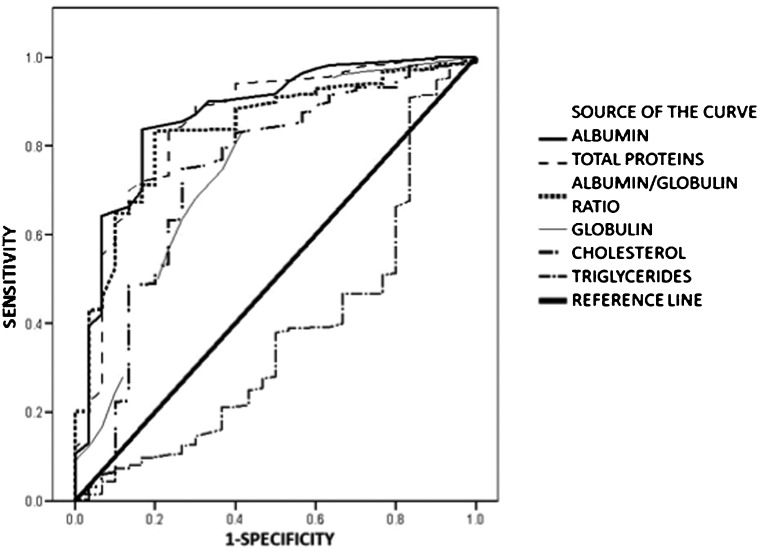
Table 3 presents the results of a univariate analysis of the qualitative data. No statistically significant difference was observed between patients younger and older than 40 years or in relation to gender, marital status, or occupation. The highest mortality was observed in the following cases: the burn causative agent was flame, full-thickness burns, the injury resulted from inhalation, and the serum albumin level was <2 g/dL. Table 4 presents the results of the analysis of the quantitative variables. The average affected BSA in the patients who died was 49.71±24.28% vs. 14.72±13.32% for survivors. There were also significant differences in the levels of cholesterol, TG, albumin, globulin, and total proteins, and in the albumin/globulin ratio, between the patients who survived and those who died. The levels of hemoglobin, hematocrit, lymphocytes, neutrophils, platelets, glucose, urea, or creatinine in patients who died did not differ significantly from those of the survivors. Figure 1 shows the receiver-operating characteristic (ROC) curves comparing the quantitative variables of the burn patients upon admission to the specialized management unit that differed significantly (albumin, globulin, total proteins, cholesterol, TG, and the albumin/globulin ratio). The greatest area under the ROC curve was observed for albumin (0.869), followed by serum proteins, cholesterol, and TG. Using a cut-off point of 1.95 g/dL albumin, the sensitivity of albumin in predicting mortality was 84%, and the specificity was 83.3%. For all patients, 20.6% had albumin levels <2 g/dL, 38.7% had levels of 2–3 g/dL, and 40.7% had levels >3 g/dL.
Table 3.
Univariate analysis.
| Survived | Died | p-value | OR (95% CI) | |
| Age (years) | ||||
| >40 | 283 | 19 | 0.31 | 1.42 (0.6–2.9) |
| <40 | 168 | 16 | 0.31 | |
| Gender | ||||
| Male | 350 | 23 (6.19%) | 0.10 | 1.81 (0.8–3.9) |
| Female | 101 | 12 (10.6%) | ||
| Marital status | ||||
| Married | 296 | 23 | 0.99 | 1 (0.4–2.1) |
| Other | 155 | 12 | ||
| Occupation | ||||
| Employee | 238 | 14 | 0.14 | 1.6 (0.7–3.5) |
| Other | 213 | 21 | ||
| Burn agent | ||||
| Flame | 221 | 30 | 0.003 | 2.7 (1.2–5.7) |
| Others | 230 | 5 | ||
| Full-thickness | ||||
| No | 314 | 16 | 0.004 | 2.7 (1.2–5.7) |
| Yes | 137 | 19 | ||
| Inhalation burns | ||||
| No | 406 | 19 | 0.000 | 7.6 (3.4–16.8) |
| Yes | 45 | 16 | ||
| Serum albumin | ||||
| <2 g/dL | 71 | 29 | 0.000 | 25.8 (9.7–72.3) |
| >2 g/dL | 380 | 6 |
OR, odds ratio; 95% CI, 95% confidence interval.
Table 4.
Analysis of quantitative variables.
| Survivors (n = 451) | Deceased patients (n = 35) | *)p-value | |
| Age (years) | 38.7±16.6 | 41.9±16.6 | 0.269 |
| Body surface burned (%) | 14.7±13.3 | 49.7±24.4 | 0.000 |
| ABSI at admission | 5.0±1.8 | 9.3±2.5 | 0.000 |
| Hemoglobin | 14.2±2.4 | 13.9±4.4 | 0.672 |
| Hematocrit | 40.7±6.4 | 39.9±11.9 | 0.697 |
| Leucocytes | 15,823.2±17,985.8 | 18,068.2±11,717.1 | 0.468 |
| Total lymphocytes | 2,582.6±4,155.4 | 2,130.9±2,953.7 | 0.529 |
| Total neutrophils | 10,741.5±11,661.6 | 12,316.7±9,917.5 | 0.437 |
| Platelets | 240,266±90,843.4 | 257,800±154,504.6 | 0.512 |
| Glucose | 124.0±91.2 | 145.2±76.3 | 0.181 |
| Urea | 41.9±83.2 | 64.7±103.3 | 0.212 |
| Creatinine | 0.9±1.0 | 1.1±1.2 | 0.273 |
| TC | 144.5±71.6 | 111.3±54.6 | 0.011 |
| TG | 144.7±101.0 | 187.5±152.5 | 0.032 |
| Globulin | 2.8±0.5 | 1.5±0.6 | 0.000 |
| Albumin | 2.8±0.8 | 1.5±0.7 | 0.000 |
| TP | 5.6±1.1 | 3.7±1.2 | 0.000 |
p-value according to Student'st-test.
ABSI, Abbreviated Burn Severity Index; TC, total cholesterol; TG, triglycerides; TP, total proteins.
Figure 1.
Receiver-operating characteristic (ROC) curves. Albumin = 0.861, total protein = 0.853, albumin/globulin ratio = 0.837, globulin = 0.755, cholesterol = 0.734, and triglycerides = 0.373.
The mean hospital stay was 25.2±30.7 days (range, 4–251 days). Forty-five patients required ventilatory support because of inhalation injury or progressive respiratory failure caused by multiple organ failure, hypovolemic shock, or massive pulmonary embolism. The average intensive care unit stay of patients with ventilatory support was 38.9 days. Early mortality (<7 days) was observed in 13 patients and attributed to multiple organ failure or hypovolemic shock. These patients all suffered inhalation burns.
Late mortality was observed in 22 patients, 12 of whom died of multiple organ failure, eight of progressive respiratory failure, and the remaining two as a consequence of massive pulmonary embolism.
DISCUSSION
The overall mortality in our series was 7.2%. According to the ABSI scores at admission, mortality was overestimated for the patients with ABSI scores of 4–5, 8–9, and >12. Our results contrast with those reported by Lionelli et al., who found that the ABSI score was strongly predictive for mortality (p<0.0001), with a 200% average increase in mortality per unit increase in ABSI (12). Therefore, the expected values overestimate the actual mortality values. To address these discrepancies, some investigators have sought the prognostic value of different scales by simplifying them (13–17) or by increasing the difficulty of obtaining results with more complex calculations (18–20). Because the most important function of albumin is to maintain an oncotic pressure of at least 80% of the normal level, its reduction could induce complications that predispose patients to malnutrition, the loss of immune responses, and an increased risk of infection (21). Several studies have shown the deleterious effects of hypoalbuminemia in terms of hospital stay, morbidity, and mortality in patients with various conditions, including critically ill patients or those patients undergoing chronic renal replacement therapy and surgical patients (22–24). Recently, Ramos et al. (25) found that severe hypoalbuminemia (<2 g/dL), which was observed in 15 of the 73 burn patients (20%), was strongly associated with an increase in the affected BSA (p<0.001), greater burn severity (according to the Benaim classification, p<0.001), and a higher mortality rate (33% vs. 0%). These authors concluded that serum albumin level can be used as a marker for trauma severity and as a biological marker for the different stages of evolution of severely burned patients. In contrast, in a cross-sectional study with fewer patients, Miquet-Romero et al. reported a mortality rate of <10% in severely burn patients (2/23) in whom hypoalbuminemia was frequently observed, demonstrating a significant association between the extent of the burn and the serum albumin level. However, these authors found no relationship between the level of this protein and hospital stay, complication rate, or mortality (26). Despite the conflicting views of the previously mentioned studies, Kim et al. (who conducted a cross-sectional analysis of 147 burn patients) showed that albumin level predicted not only mortality but also the risk of developing kidney failure, which eventually resulted in 100% mortality in the 28 patients who developed this complication (27). Eljaiek and Dubois performed another cross-sectional study of 56 burned patients with a total burn size (TBS) of 30.3±10.9%, 36 (64.3%) patients had hypoalbuminemia (<30 g/L) and 20 (35.7%) had albumin levels >30 g/L (28). The authors demonstrated that the presence of hypoalbuminemia in the first 24 hours after injury was an independent predictor of organ dysfunction. They observed no mortality in the patients with normal albumin levels but 16.7% mortality in patients with hypoalbuminemia. However, the difference was not statistically significant, likely because the sample size was small.
We also examined the relationships between mortality and the levels of other serum proteins and lipids. The sensitivity and specificity of total serum proteins, globulin, the albumin/globulin ratio, cholesterol, and TG were inferior to those of albumin in predicting mortality. In another cross-sectional study, Papadopoulos et al. (29) demonstrated that the mean cholesterol levelof the patients who died was lower than that of the surviving patients when the TBS was >30%. However, the authors did not evaluate the relationship between serum proteins and mortality.
Our data showed that albumin levels may be useful in predicting mortality in burn patients. These skin injuries induce a strong inflammatory response and the release of vasoactive substances, which increases the skin permeability to water, albumin, and even larger protein molecules (21,30–32). This process peaks during the first 12–24 hours, coupled with a reduction in the albumin mRNA response to the burn and an increased synthesis and accumulation of acute-phase reactants. After this initial phase, hypoalbuminemia persists because of the lack of its synthesis, its loss through damaged skin areas, and deficiencies in nutritional support (33–35). In our patients, hypoalbuminemia was closely related to the burned BSA, which is considered to have predictive value for mortality. Both events are closely related. The albumin level is the only modifiable factor related to outcomes because albumin levels can be corrected by administering human albumin solutions (HAS), which have been used for either fluid resuscitation or hypoalbuminemia correction. Few studies have assessed the use of HAS for fluid resuscitation in severely burned patients. Initially, a meta-analysis showed increased mortality in the burn patients resuscitated with HAS (36–39). Melinyshyn et al. (40) recently published the results of a cross-sectional study of two groups of burn patients treated with either albumin infusions to maintain their serum albumin concentrations above 2 g/L or with standard solutions after the resuscitation phase. The authors could not demonstrate any difference in the wound healing, length of hospital stay, or mortality. They concluded that routine supplementation with 5% HAS to maintain the serum albumin levels of burn patients above 2 g/L was expensive and provided no extra benefit.
Another clinical trial and a systematic review showed neutral effects (41–45). However, the efficacy of hypoalbuminemia correction can be extrapolated from studies performed in other types of critically ill patients. A recent meta-analysis found that using albumin-containing solutions to resuscitate septic patients was associated with lower mortality than other fluid resuscitation treatments (46).
In addition to its well-known oncotic properties, albumin has many other physiological roles that might support hypoalbuminemia correction in critically ill patients, such as the binding of endogenous and exogenous substances, anticoagulant effects, maintenance of acid–base status, modulation of apoptosis, and protective effects on the microcirculation (47).
Another issue to consider is the role of nutritional support. The best possible nutrition and nutritional route for each individual must be guaranteed. Enteral nutrition is preferable, but if this treatment cannot provide the minimum requirements to halt the catabolic phase, induce the anabolic phase, or cannot be used for any reason, then the parenteral route or combined enteral/parenteral routes should be used to ensure the provision of macronutrients (specifically proteins) in higher than normal quantities (36).
For this strategy to succeed, the modulation of the inflammatory response, immune strengthening, and the optimal management of burned skin extensions is required in addition to nutritional support to avoid immediate and late protein leakage.
Our study suggests that hypoalbuminemia has a deleterious effect on patient survival but does have some limitations. Therefore, we suggest that a prospective cohort study be conducted to confirm the association between hypoalbuminemia and the risk of death and that more multicenter prospective clinical trials be performed to establish the benefits of HAS infusion in the early and late phases of burn patient management. The outcome variables should include not only the main outcome variable of mortality and the presence or absence of infections and organ failure but also the inflammatory response.
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Dirección General de Información en Salud (DGIS) Base de datos de egresos hospitalarios por mortalidad en instituciones públicas, 2004–2007 (on line): Sistema Nacional de Información en Salud (SINAIS) (México): Secretaria de Salud. http://www.sinais.salud.gob.mx/basesdedatos/eh_sectorial_morta.html (Accesed Jan 9, 2013).
- 2.Dirección General de Información en Salud (DGIS) Base de datos de egresos hospitalarios por morbilidad en instituciones públicas, 2004–2007 (on line): Sistema Nacional de Información en Salud (SINAIS) (México): Secretaria de Salud. http://www.sinais.salud.gob.mx/basesdedatos/eh_sectorial_morbi.html (Accesed Jan 9, 2013).
- 3.Moscoso-Maza V, Cuenca-Pardo J, Álvarez-Díaz C. Análisis de la morbi-mortalidad del quemado extenso adulto. Cir Plast. 2002;12(2):71–3. [Google Scholar]
- 4.Colleen M. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;335(5):362–7. doi: 10.1056/NEJM199802053380604. [DOI] [PubMed] [Google Scholar]
- 5.Raff T, German G, Barthold U. Factors influencing the early prediction of outcome from burns. Acta Chir Plast. 1996;38(4):122–7. [PubMed] [Google Scholar]
- 6.Tobiasen J, Hiebert JM, Edlich RF. The Abbreviated Burn Severity Index. Ann Emerg Med. 1982;11(5):260–2. doi: 10.1016/s0196-0644(82)80096-6. [DOI] [PubMed] [Google Scholar]
- 7.Hörbrand F, Schrank C, Henckel-Donnersmarck G, Mühlbauer W. Integration of preexisting diseases and risk factors in the Abbreviated Burn Severity Index (ABSI) Anasthesiol Intensivmed Notfallmed Schmerzther. 2003;38(3):151–7. doi: 10.1055/s-2003-37773. [DOI] [PubMed] [Google Scholar]
- 8.Cartwright M. The metabolic response to stress: a case of complex nutrition support management. Crit Care Nur Clin North Am. 2004;16(4):467–87. doi: 10.1016/j.ccell.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Budagov RS, Ul'ianova LP. Some consequences of systemic inflammatory response in the pathogenesis of aggravation of outcomes of combined radiation and thermal injuries. Radiats Biol Radioecol. 2005;45(2):191–5. [PubMed] [Google Scholar]
- 10.Lehnhardt M, Jafari HJ, Druecke D, Steinstraesser L, Steinau HU, Klatte W, et al. A qualitative and quantitative analysis of protein loss in human burn wounds. Burns. 2005;31(2):159–67. doi: 10.1016/j.burns.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Gibran NS, Heimbach DM. Management of the patient with thermal injuries. In: Souba W W, Fink M P, Jurkovich G J, Kaiser L R, Pearce W H, Perbertom JH & Soper N J, editors. ACS Surgery. Principles and Practice. New York: WebMD; 2006. pp. 1295–306. [Google Scholar]
- 12.Lionelli GT, Pickus EJ, Beckum OK, DeCoursey RL, Korentager RA. A three decade analysis of factors affecting burn mortality in the elderly. Burns. 2005;31(8):958–63. doi: 10.1016/j.burns.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: extending and updating the Baux score. J Trauma. 2010;68(3):690–7. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 14.Smith DL, Cairns BA, Ramadan F, Dalston JS, Fakhry SM, Rutledge R, et al. Effect of inhalation injury, burn size, and age on mortality: a study of 1447 consecutive burn patients. J Trauma. 1994;37(4):655–9. doi: 10.1097/00005373-199410000-00021. [DOI] [PubMed] [Google Scholar]
- 15.Choiniére M, Dumont M, Papillon J, Garrel DR. Prediction of death in patients with burns. Lancet. 1999;353(9171):2211–2. doi: 10.1016/S0140-6736(99)02401-0. [DOI] [PubMed] [Google Scholar]
- 16.Tejero-Trujeque R. How effective is the Abbreviated Burn Severity Index in predicting patient mortality. J Wound Care. 2000;9(10):475–8. doi: 10.12968/jowc.2000.9.10.26355. [DOI] [PubMed] [Google Scholar]
- 17.Moreau AR, Westfall PH, Cancio LC, Mason AD Jr. Development and validation of an age-risk score for mortality predication after thermal injury. J Trauma. 2005;58(5):967–72. doi: 10.1097/01.ta.0000162729.24548.00. [DOI] [PubMed] [Google Scholar]
- 18.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100(6):1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 19.Pavoni V, Gianesello L, Paparella L, Buoninsegni LT, Barboni E. Outcome predictors and quality of life of severe burn patients admitted to intensive care unit. Scand J Trauma Resusc Emerg Med. 2010;18:24. doi: 10.1186/1757-7241-18-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moore EC, Pilcher DV, Bailey MJ, Cleland H, McNamee J. A simple tool for mortality prediction in burns patients: APACHE III score and FTSA. Burns. 2010;36(7):1086–91. doi: 10.1016/j.burns.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Kumar P, D'Souza J, Bhaskara KG, Bharadwaj S. Serum protein level in conjunction with serum albumin/globulin ratio as an indicator of severity of changes in capillary permeability. Burns. 2003;29(6):628–9. doi: 10.1016/s0305-4179(03)00149-9. [DOI] [PubMed] [Google Scholar]
- 22.Goldwasser P, Feldman J. Association of serum albumin in mortality risk. J Clin Epidemiol. 1997;50(6):693–703. doi: 10.1016/s0895-4356(97)00015-2. [DOI] [PubMed] [Google Scholar]
- 23.Gibbs J, Cull W, Henderson W, Daley J, Hur K, Khuri SF. Preoperative serum albumin level as a predictor of operative mortality and morbidity. Arch Surg. 1999;134(1):36–42. doi: 10.1001/archsurg.134.1.36. [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Rodriguez M, Medina-Cuadros M, Gómez-Ortega A, Martínez-Gallegos G, Mariscal-Ortiz M, Martínez-Gonzalez MA, et al. Cholesterol and serum albumin levels as predictors of cross infection, death, and length of hospital stay. Arch Surg. 2002;137(7):805–12. doi: 10.1001/archsurg.137.7.805. [DOI] [PubMed] [Google Scholar]
- 25.Ramos GE, Bolgiani A, Guastavino P, Prezzavento P, Patiño O, Benaim F. Hypoalbuminemia in burned patients: An outcome marker that could define evolution periods. Rev Arg Quem. 2000;15(1):20–8. [Google Scholar]
- 26.Miquet-Rodríguez LM, Rodríguez-Garcell R, Santana-Porben S, Cervantes-Flores R. Valor Pronóstico del nivel de albúmina sérica inicial en los pacientes quemados. http://www.portalesmedicos.com/publicaciones/articles/1108/2/Valor-pronostico-del-nivel-de-albumina-serica-inicial-en-los-pacientes-quemados (Accessed Jan 9, 2013). [Google Scholar]
- 27.Kim GH, Oh KW, Yoon JW, Koo JR, Kim HJ, Chae DW, et al. Impact of burn size and initial serum albumin level on acute renal failure occurring in major burn. Am J Nephrol. 2003;23(1):55–60. doi: 10.1159/000066299. [DOI] [PubMed] [Google Scholar]
- 28.Eljaiek R, Dubois MJ. Hypoalbuminemia in the first 24 h of admission is associated with organ dysfunction in burned patients. Burns. 2013;39(1):113–8. doi: 10.1016/j.burns.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 29.Papadópulos-Canales AA, Gutierrez-Salgado E, Duffy-Verrdura BE, Fernández-Sobrino G, Portal-Celhay C, Pérez-Penilla F. Hipocolesterolemia y evolución clínica en pacientes quemados graves. Cir Plast. 2005;15(3):140–4. [Google Scholar]
- 30.Arturson G. Pathophysiology of the burn wound and pharmacological treatment. The Rudi Hermans Lecture, 1995. Burns. 1996;22(4):255–74. doi: 10.1016/0305-4179(95)00153-0. [DOI] [PubMed] [Google Scholar]
- 31.Birke G, Liljedahl SO, Plantin LO, Reizenstein P. Studies on burns. IX. The distribution and losses through the wound of 131I-albumin measured by whole-body counting. Acta Chir Scand. 1968;134(1):27–36. [PubMed] [Google Scholar]
- 32.Brouhard BH, Carvajal HF, Linares HA. Burn edema and protein leakage in the rat. I. Relationship to time of injury. Microvasc Res. 1978;15(2):221–8. doi: 10.1016/0026-2862(78)90020-1. [DOI] [PubMed] [Google Scholar]
- 33.Kumar P. Grading of severity of the condition in burn patients by serum protein and albumin/globulin studies. Ann Plast Surg. 2010;65(1):74–9. doi: 10.1097/SAP.0b013e3181c47d71. [DOI] [PubMed] [Google Scholar]
- 34.Dickson PW, Bannister D, Schreiber G. Minor burns lead to major changes in synthesis rates of plasma proteins in the liver. J Trauma. 1987;27(3):283–6. doi: 10.1097/00005373-198703000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Sevaljevic L, Ivanovic-Matic S, Petrovic M, Glibetic M, Pantelic D, Poznanovic G. Regulation of plasma acute-phase protein and albumin levels in the liver of scalded rats. Biochem J. 1989;258(3):663–8. doi: 10.1042/bj2580663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guastavino MP, Schulberg-Pizano L, Ramos G, Benaim F. Nutritional support and hypoalbuminemia in critical burned patients. Rev Arg Quem. 2000;15(30):6–7. [Google Scholar]
- 37.Recinos PR, Hartford CA, Ziffren SE. Fluid resuscitation of burn patients comparing a crystalloid with a colloid containing solution: a prospective study. J Iowa Med Soc. 1975;65:426–32. [PubMed] [Google Scholar]
- 38.Cochrane Injuries Group Albumin Reviewers. Human albumin administration in critically ill patients: systematic review and randomized controlled trials. BMJ. 1998;317(7153):235–40. doi: 10.1136/bmj.317.7153.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godwin CW, Dorethy J, Lam V, Pruitt BA., Jr Randomized trial of efficacy of crystalloid and colloid resuscitation on hemodynamic response and lung water following thermal injury. Ann Surg. 1983;197(5):520–31. doi: 10.1097/00000658-198305000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melinyshyn A, Callum J, Jeschke MC, Cartotto R. Albumin supplementation for hypoalbuminemia following burns: unnecessary and costly! J Burn Care Res. 2013;34(1):8–17. doi: 10.1097/BCR.0b013e31825f3186. [DOI] [PubMed] [Google Scholar]
- 41.JelenkoC, Wheeler ML, Callaway BD, Divilio LT, Bucklen KR, Holdredge TD. Shock and resuscitation. II: Volume repletion with minimal edema using the “HALFD” (Hypertonic Albuminated Fluid Demand) regimen. JACEP. 1978;7(9):326–33. doi: 10.1016/s0361-1124(78)80356-6. [DOI] [PubMed] [Google Scholar]
- 42.Greenhalgh DG, Housinger TA, Kagan RJ, Rieman M, James L, Novak S, et al. Maintenance of serum albumin levels in pediatric burn patients: a prospective, randomized trial. J Trauma. 1995;39:67–73. doi: 10.1097/00005373-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Cooper AB, Cohn SM, Zhang HS, Hanna K, Stewart TE, Slutsky AS. Five percent albumin for adult burn shock resuscitation: lack of effect on daily multiple organ dysfunction score. Transfusion. 2006;46(1):80–9. doi: 10.1111/j.1537-2995.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 44.Finfer S, Bellomo R, Boyce N, French J, Myburgh J, Norton R. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350(22):2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 45.Dubois MJ, Heylbroeck C, Deroy P, Bracco D, Burns KE, Sirdar E, et al. Administration of albumin in burn patients: a systematic review. J Burn Care Res. 2007;28:S102. [Google Scholar]
- 46.Delaney AP, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: a systematic review and meta-analysis. Crit Care Med. 2011;39(7):386–91. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 47.Dubois MJ, Vincent JL. Use of albumin in the intensive care unit. Curr Opin Crit Care. 2002;8:299–301. doi: 10.1097/00075198-200208000-00005. [DOI] [PubMed] [Google Scholar]