Figure 4.
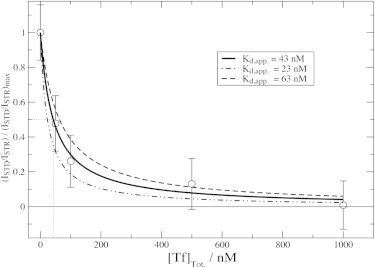
1D STD monitored isotherm for the Aβ (12–28) self-association inhibition by Tf. Tf was titrated into a solution of salt-aggregated 650 μM Aβ (12–28) and 1D STD and STR spectra were acquired at 293 K and at 600 MHz using a Bruker TBI-Z probe. Experimental data points are reported as circles, whereas the solid line was obtained through nonlinear fitting of the equation f + (1-f)∗Kd,app./(Kd,app. + [Tf]), where f is the fraction of Aβ (12–28) oligomers that are not Tf-binding competent, [Tf] is the concentration of free Tf and Kd,app. is an apparent average dissociation constant for the complexes between Tf and the other Aβ (12–28) oligomers (1). The dashed and dot-dashed lines were obtained using the same equation but by varying the Kd,app. value obtained through nonlinear curve fitting (i.e., 43 nM, assuming that the product of the total concentration of Tf-binding competent Aβ oligomers and of the ratio of the number of Tf molecules bound per Aβ oligomer versus the number of Aβ oligomers bound per Tf molecule is ≤ 5 nM. If this product is >5 nM, the Kd,app. values reported in the figure become just an upper limit to the best fit Kd,app. values). We also assumed that all Tf molecules bind to equivalent and independent binding sites (Scatchard-like model) (1). The latter assumption reflects the simplest model that fits the experimental data. The dotted line defines the IC50 for the inhibition of Aβ (12–28) self-association inhibition by Tf.
