Abstract
Background
Reduced density of capillaries in skeletal muscle can limit insulin, glucose, and oxygen supply to the muscle, thereby contributing to worsening metabolism in older adults. The lower skeletal muscle capillarization in impaired glucose tolerance (IGT) may partially be due to circulating angiogenic cell dysfunction. Circulating angiogenic cells maintain the vasculature and promote angiogenesis, but circulating angiogenic cell number and function may be reduced in IGT. The goal of this study was to determine whether the clonogenic potential of circulating angiogenic cells is lower in IGT compared with normal-glucose-tolerant (NGT) controls and is associated with skeletal muscle capillarization.
Methods
Glucose tolerance, endothelial cell colony-forming unit (CFU-EC) number, and vastus lateralis capillary density were measured in sedentary, older (62±1 years, mean±SEM) men and women with NGT (n=16) and IGT (n=12).
Results
Adults with IGT had 43% lower CFU-EC number (11.4±2.3 versus 20.1±2.0 colonies, p<0.01) and 12% lower capillary density (291±11 versus 330±9 capillaries/mm2, p<0.01) compared with those with NGT. In regression analyses, CFU-EC number inversely correlated with 120-min postprandial glucose in all subjects (r=−0.47, p<0.05), and capillary density was directly associated with CFU-EC number (r=0.53, p<0.05).
Conclusions
We conclude that the clonogenic potential of circulating angiogenic cells is lower in sedentary older adults with IGT and is associated with lower skeletal muscle capillarization. Low circulating angiogenic cell clonogenic potential in IGT suggests a state of impaired angiogenesis occurring prior to overt type 2 diabetes that may mediate early microvascular changes in the development and progression of IGT to type 2 diabetes. Published 2013. This article is a U.S. Government work and is in the public domain in the USA.
Keywords: type 2 diabetes, insulin resistance, endothelium, endothelial cell, angiogenesis
Introduction
Insulin resistance and type 2 diabetes are associated with vascular complications including reduced endothelial vasoreactivity, impaired angiogenesis, and microvasculopathy. There is a continuum of glucose intolerance ranging from normal glucose tolerance (NGT) to type 2 diabetes, which is associated with progressive worsening of vascular function and health [1]. We recently reported that otherwise healthy older adults with impaired glucose tolerance (IGT) have lower skeletal muscle capillarization compared with NGT controls [2], showing that diabetes-associated capillary rarefaction occurs prior to the development of overt type 2 diabetes. Lower capillarization in the skeletal muscle of older adults with IGT is detrimental because transcapillary delivery of metabolic substrates and hormones can be limited when there are fewer capillaries to perfuse [3]. Transcapillary transport of insulin is an important determinant of glucose uptake in the skeletal muscle [4]; therefore, a reduction in capillarization could contribute to further impairments in glucose metabolism in subjects with IGT.
Circulating angiogenic cells (CACs) are a population of circulating cells composed of endothelial progenitor cells (EPCs) and other pro-angiogenic cell types that contribute to angiogenesis and maintenance of the vasculature in vivo [5]. Thus, impaired function of CACs is one potential mechanism underlying vascular dysfunction and microvascular rarefaction in IGT. Previous reports show impaired function and lower numbers of certain CAC subtypes in type 2 diabetes [6–9] and that circulating EPC number inversely correlates with glucose tolerance in a range of subjects with and without type 2 diabetes [7]. Less is known about potential impairments in CAC function in glucose intolerance; therefore, we sought to determine whether CAC clonogenic potential (i.e. the ability of CACs to generate endothelial cell colonies) is lower in IGT by measuring ex vivo endothelial cell colony-forming unit (CFU-EC) number. The CFU-EC number is inversely associated with Framingham cardiovascular risk score and is directly associated with in vivo vascular function measured by flow-mediated brachial artery reactivity [10]. Because CAC dysfunction may occur prior to overt type 2 diabetes and is associated with vascular dysfunction, we hypothesized that CFU-EC number is lower in adults with IGT compared with those with NGT and is associated with skeletal muscle capillarization.
Materials and methods
Subjects
Twenty-eight sedentary (self-reported exercise less than 20 min on two or fewer days per week), older (51–73 years) men and women who were non-smokers and had no previous diagnosis of diabetes or cardiovascular disease participated in this study. This study was approved by the Institutional Review Board at the University of Maryland School of Medicine, and all subjects provided written informed consent.
CFU-EC assay
CFU-EC number was assessed using the CFU-Hill assay (StemCell Technologies, Vancouver, Canada). Fasting blood samples were obtained, and peripheral blood mononuclear cells were isolated by density gradient centrifugation. The cells were washed twice with PBS supplemented with 2% FBS and plated at 5×106 cells/well on six-well culture plates coated with human fibronectin (BD Pharmingen, Franklin Lakes, NJ) in 2 mL Endocult Medium (Stem Cell Technologies, Vancouver, BC). Cells were incubated at 37 °C, 5% CO2, and after 48 h, non-adherent cells were harvested and replated at 1×106 cells/well on 24-well fibronectin-coated plates (BD Pharmingen) in 1 mL Endocult Medium. CFU-ECs were counted 72 h later and were defined according to previously established methodology where colonies are identified as having central cores of round cells with more elongated sprouting cells at the periphery (10). Technicians blinded to the status of the sample counted CFU-EC number; the mean of four randomly selected wells was used in the analyses.
Oral glucose tolerance test
The subjects underwent a 2-h oral glucose tolerance test after a 12-h overnight fast. A catheter was placed in an antecubital vein, and blood samples were drawn before and 120 min after the ingestion of a 75-g glucose solution. Blood samples were centrifuged, and plasma was separated and stored at −80 °C until analysis. Plasma glucose levels were analyzed with a glucose analyzer (2300 STAT Plus, YSI, Yellow Springs, OH). Plasma insulin levels were determined by radioimmunoassay (Millipore, St. Charles, MO). Subjects were classified as having NGT or IGT by the American Diabetes Association criteria (NGT: fasting plasma glucose of <5.6 mmol/L and 2-h postprandial glucose <7.8 mmol/L; IGT: fasting plasma glucose of <7.0 mmol/L and 2-h postprandial glucose 7.8–11.0 mmol/L) [11]. The homeostatic model assessment for insulin resistance (HOMA-IR) was calculated as described by Matthews et al. [12].
Muscle biopsies
Percutaneous needle biopsies were obtained from the vastus lateralis, approximately 12–13 cm above the patella on the anterolateral aspect of the thigh using a Bergstrom needle (Stille, Solna, Sweden) as previously described [13]. Muscle samples were rapidly embedded in OCT-tragacanth gum mixture, frozen, and stored at −80 °C for histochemical analyses.
Capillary density
Muscle was sectioned to a thickness of 14 μm on a cryostat, and capillaries were identified with a modified double stain technique [14]. Immunostained muscle sections were viewed under a light microscope, and digital images were obtained (Eclipse Ti, Nikon Instruments Inc., Melville, NY). Quantification of capillarization was performed on at least 50 fibres for each sample (mean=73±3 fibres/sample); sampling a larger number of fibres does not improve the estimation of capillarization in human muscle [15]. Images were analyzed by a technician blinded to sample status using NIS Elements software (Nikon Instruments Inc., Melville, NY). The following five indices of capillarization were measured: (1) capillary contacts (the number of capillaries in contact with each muscle fibre), (2) share factor (the number of fibres that share each capillary), (3) capillary-to-fibre ratio (the number of whole capillary equivalents in contact with each muscle fibre), (4) capillary density (CD: the number of capillaries per square millimetre of muscle cross-sectional area), and (5) capillary-to-fibre perimeter exchange index (CFPE: the number of capillaries per millimetre of muscle fibre perimeter).
Body composition
Body composition (fat mass, fat-free mass, lean body mass, and percent body fat) was determined by dual energy X-ray absorptiometry (Prodigy, LUNAR Radiation Corp., Madison, WI). Waist circumference was measured using a tape measure; three measurements were taken and then averaged for use in analyses.
Blood pressure and lipoprotein-lipid profiles
Blood pressure measurements were obtained by auscultation after the subjects were seated comfortably for 15 min with the arm supported at heart level. Fasting plasma total cholesterol, high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), and triglyceride levels were determined using conventional methods as previously described [16]. Samples were obtained after a 12-h overnight fast on three separate days and averaged for use in analyses.
Maximal oxygen consumption
Maximal oxygen consumption (VO2max) was measured by indirect calorimetry during a graded treadmill exercise test on a motorized treadmill as previously described [17]. Briefly, subjects walked at a constant velocity throughout the protocol; grade was initially set to 0% and increased every 2 min thereafter to maximal effort. VO2max was defined as the highest oxygen consumption value obtained for a full 30-s increment. Attainment of VO2max was verified by standard physiological criteria (respiratory exchange ratio >1.10 or a plateau in VO2 with an increase in workload).
Statistical analyses
All analyses were performed using SPSS v12.0 (IBM, Armonk, NY). Data are presented as means±SEM. Analysis of covariance was used to test for differences in outcome variables; sex and race were used as covariates where appropriate. A regression analysis using an inverse model was used to test for associations between 120-min postprandial glucose and CFU-EC number; multivariable linear regression analysis was used to test for associations between CFU-EC number and CD and CFPE. Chi-square analysis was used to test for differences in distribution of race, sex, and medication use between groups (IGT and NGT). A type I error rate of α=0.05 was selected, and two-tailed probabilities are reported for all analyses.
Results
Subject characteristics are shown in Table 1. Of the 28 subjects in this study, 16 had NGT and 12 had IGT. As expected, subjects with IGT had significantly higher 120-min postprandial glucose (p<0.001), higher 120-min postprandial insulin (p=0.001), and higher HOMA-IR values (p<0.05) than their NGT counterparts. In addition, subjects with IGT had higher fasting plasma glucose (p<0.05) and tended to have higher fasting plasma insulin concentrations (p=0.07) compared with those with NGT. Subjects with IGT tended to have lower VO2max compared with those with NGT when expressed relative to body weight (mL/kg/min, p=0.09), but this difference was eliminated when expressing VO2max relative to lean body mass (mL/kgLBM/min, p=0.46). There were no statistically significant differences in age, weight, BMI, body composition, or waist circumference between subjects with IGT and NGT. Subjects in both groups had similar HDL-C and triglyceride levels, but subjects with IGT had lower total cholesterol and LDL-C levels compared with those with NGT. Although not statistically significant (p=0.17), a higher proportion of subjects in the IGT group (6 of 12 subjects or 50%) were taking statins prescribed for hyperlipidemia compared with the NGT group (4 of 16 subjects or 25%). The distribution of race and sex did not differ significantly between groups; however, sex and race were evaluated for effects on our primary outcome measures (120-min postprandial glucose, CFU-EC number, and CD). There was an independent effect of race on CD, with CD being higher in white subjects compared with black subjects (335±12 versus 292±12, respectively, p=0.02), but the NGT and IGT groups were balanced with respect to race. There were no other statistically significant main effects or sex * race interactions on our primary outcomes (p=0.12–0.86); however, sex and race were evaluated as covariates in subsequent analyses.
Table 1.
Subject characteristics and plasma glucose levels in older adults with normal (NGT) and impaired (IGT) glucose tolerance
| NGT (n = 16) | IGT (n = 12) | |
|---|---|---|
| Sex: Women/men (number) | 10/6 | 9/3 |
| Race: Black/White (number) | 8/8 | 6/6 |
| Age (years) | 62±1 | 64±2 |
| Weight (kg) | 90.9±4.3 | 91.0±5.0 |
| BMI (kg/m2) | 32.4±1.5 | 33.2±1.2 |
| Body fat (%) | 40.2±1.3 | 42.2±1.5 |
| Fat mass (kg) | 38.8±2.7 | 39.5±3.3 |
| Fat-free mass (kg) | 54.1±1.6 | 52.4±2.0 |
| Waist circumference (cm) | 102±2 | 106±3 |
| Systolic blood pressure (mmHg) | 121±2 | 121±2 |
| Diastolic blood pressure (mmHg) | 77±2 | 78±2 |
| Total cholesterol (mmol/L) | 5.2±0.2 | 4.4±0.2* |
| LDL-C (mmol/L) | 3.2±0.2 | 2.6±0.2* |
| HDL-C (mmol/L) | 1.3±0.1 | 1.2±0.1 |
| Triglycerides (mmol/L) | 1.3±0.1 | 1.3±0.1 |
| VO2max (L/min) | 2.47±0.12 | 2.26±0.13 |
| VO2max (mL/kg/min) | 26.1±1.1 | 23.5±1.2 |
| VO2max (mL/kgLBM/min) | 48.2±2.2 | 46.0±2.5 |
| Fasting plasma glucose (mmol/L) | 5.0±0.2 | 5.7±0.2* |
| 120-min postprandial glucose (mmol/L) | 5.6±0.2 | 9.9±0.5** |
| Fasting plasma insulin (pmol/L) | 108±12 | 142±15 |
| 120-min postprandial insulin (pmol/L) | 436±77 | 883±91** |
| HOMA-IR | 3.6±0.5 | 5.3±0.6* |
Data are presented as means±SEM.
There was a tendency for fasting plasma insulin levels to be higher in subjects with IGT (p = 0.07).
HDL-C, high-density lipoprotein-cholesterol; HOMA-IR, homeostatic model assessment for insulin resistance; LDL-C, low-density lipoprotein-cholesterol.
Significant difference between groups:
p<0.05,
p≤0.001.
Concordant with our hypothesis, CFU-EC number was 43% lower in subjects with IGT compared with those with NGT (11.4±2.3 versus 20.1±2.0, p < 0.01, Figure 1) in an analysis of covariance adjusting for race (covariate effect of race, p=0.01). In the regression analysis, CFU-EC number was inversely associated with 120-min postprandial glucose level in all subjects across the continuum of glucose tolerance (r=−0.47, p<0.01). CFU-EC number was not associated with fasting plasma glucose (r=0.05, p=n.s.), nor with body composition, VO2max, plasma lipoprotein–lipid levels, or insulin concentrations (r=−0.08–0.19, p 0.38 for all). There was no significant effect of statin use on CFU-EC number in any analysis (p=0.62–0.93).
Figure 1.
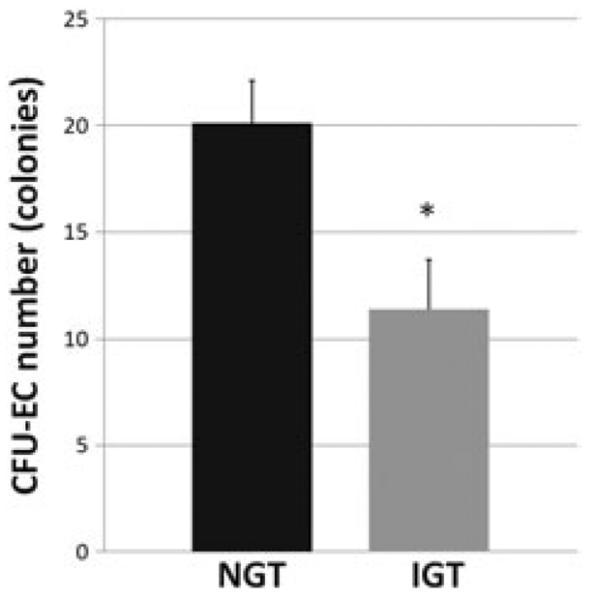
The number of endothelial cell colony-forming units (CFU-ECs) in subjects with normal (NGT) and impaired (IGT) glucose tolerance. Data are presented as means ± SEM. *Significant difference between groups: p < 0.01.
Skeletal muscle capillarization and fibre size data are presented in Table 2. Compared with subjects with NGT, those with IGT had significantly lower CFPE (p<0.05) and CD (p<0.01) in an analysis of covariance adjusting for sex and race (covariate effects, p=0.02–0.04). Although capillary contacts and capillary-to-fibre ratio also appeared lower in IGT compared with NGT, these did not reach statistical significance. Importantly, we found no differences in muscle fibre area or perimeter between IGT and NGT groups; therefore, the differences in CD and CFPE are not attributable to differences in muscle fibre cross-sectional area. In a multivariable regression analysis accounting for sex, CFU-EC number was directly associated with CD in all subjects (CFU-EC partial r=0.53, p<0.05; model r=0.56, p<0.01, Figure 2). Likewise, CFU-EC number was directly associated with CFPE (CFU-EC partial r=0.43, p<0.05; model r=0.66, p<0.01).
Table 2.
Skeletal muscle capillarization in older adults with normal (NGT) and impaired (IGT) glucose tolerance
| NGT (n = 16) | IGT (n = 12) | |
|---|---|---|
| Skeletal muscle fibre area (μm2) | 5186±326 | 5160±391 |
| Skeletal muscle fibre perimeter (μm) | 284±9 | 285±11 |
| Share factor | 2.90±0.04 | 3.00±0.04 |
| Capillary contacts (individual capillary contacts per fibre) | 4.14±0.20 | 3.73±0.26 |
| Capillary-to-fibre ratio (whole capillary equivalents per fibre) | 1.51±0.09 | 1.32±0.10 |
| Capillary-to-fibre perimeter exchange index (capillaries/mm) | 5.45±0.15 | 4.87±0.19* |
| Capillary density (capillaries/mm2) | 330±9 | 291±11** |
Data are presented as adjusted means±SEM (sex and race included as covariates where appropriate).
Significant difference between groups:
p<0.05,
p<0.01.
Figure 2.
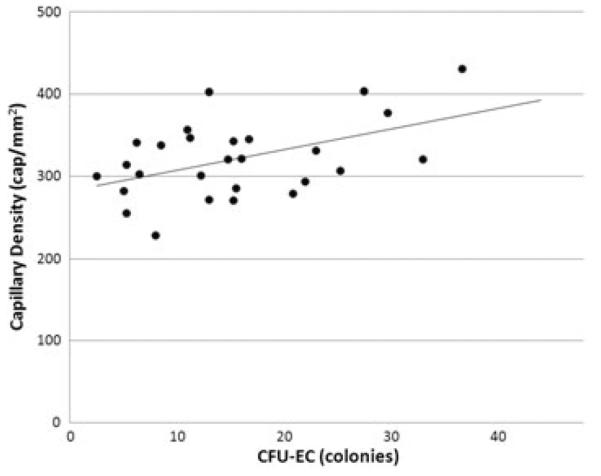
Scatterplot depicting the correlation between endothelial cell colony-forming unit (CFU-EC) number and vastus lateralis capillary density in all subjects (r = 0.53, p < 0.01).
Discussion
The novel findings from this study are that the clonogenic potential of CACs is significantly lower in older adults with IGT compared with their NGT counterparts and that lower CFU-EC number is directly associated with lower skeletal muscle capillarization in sedentary, obese, older adults across the spectrum of glucose tolerance. These findings have potential clinical importance as lower CAC function in IGT could partially mediate capillary rarefaction in skeletal muscle and contribute to worsening glucose metabolism by limiting transcapillary transport of insulin and glucose to skeletal muscle. The higher levels of plasma insulin and glucose we observed in our subjects with IGT and low skeletal muscle capillarization are consistent with this notion. Our findings may also have implications at the macrovascular level, as low CFU-EC number is associated with low endothelial vasoreactivity [10] and could contribute to the impaired endothelial function observed in IGT [18].
In the present report, we confirm our previous finding that skeletal muscle capillarization is lower in otherwise healthy subjects with IGT compared with NGT controls [2]. In an attempt to determine factors that may contribute to this reduction in capillarization, we studied an index of CAC function (CFU-EC number) and its relationship to glucose tolerance and skeletal muscle capillarization in subjects with a range of glucose tolerance from normal to impaired. It stands to reason that the clonogenic potential of putative CACs could be determined in part by CAC number as well as CAC function and proliferative potential. To date, most research on CACs in disorders of glucose metabolism has focused on putative EPCs (a subset of CACs) in subjects with type 2 diabetes. Previous studies have shown that the number and function of certain EPCs are lower in type 2 diabetes [6–9]. Li et al. [19] demonstrated lower pro-angiogenic potential of cultured EPCs from subjects with type 2 diabetes using a tube formation assay with EPCs generated in a colony-forming assay. Likewise, Tepper et al. [8] found 48% lower ex vivo EPC proliferation in subjects with type 2 diabetes compared with normal controls and that there was less incorporation of these cells into vascular structures in a murine implant model. Many of the impairments in CAC number and function in type 2 diabetes correlate with disease severity [20], suggesting a range of dysfunction that may extend to impaired glucose tolerance. Although the EPC number is lower in subjects with type 2 diabetes compared with controls, Fadini et al. [7] found that the EPC number did not differ between subjects with NGT and those with IGT and/or impaired fasting glucose. To date, we are unaware of a comprehensive enumeration of subsets of CACs in subjects with IGTor diabetes; however, if CAC number is also not lower in IGT compared with NGT, this would indicate that impairments in CAC function may be primarily responsible for the lower clonogenic potential we observe. This reduction in CAC function in IGT has the potential to contribute to microvasculopathy and impairments in endothelial vasoreactivity and angiogenesis prior to the onset of overt type 2 diabetes. Furthermore, through a reduction in skeletal muscle capillarization, impaired CAC function could contribute to the progression of type 2 diabetes by limiting insulin and glucose supply to the muscle.
Recent evidence supports several possible mechanisms that may adversely affect CAC function in older adults with IGT including inflammation, oxidative stress, and hyperglycaemia. First, there is chronic low-grade inflammation in IGT evidenced by increased circulating concentrations of C-reactive protein (CRP) [21] and tumour necrosis factor-α (TNFα) [22], and these inflammatory cytokines may contribute to the dysfunction of certain CACs [20]. For example, CRP inhibits differentiation and survival of cultured CACs at physiological concentrations [23], and TNFα inhibits EPC differentiation and proliferation in a dose-dependent manner [24]. Second, systemic and tissue-specific oxidative stress are heightened in insulin resistance and impaired glucose tolerance [25,26], and high intracellular levels of reactive oxygen species and endothelial nitric oxide synthase uncoupling occur in EPCs from patients with type 2 diabetes [9]. Also, two subsets of CACs from a murine model of diabetes were shown to be more sensitive to oxidative stress-induced cell death compared with CACs from normal mice [27]. Oxidative stress in the form of high intracellular superoxide levels may cause CAC dysfunction [28], and increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase expression and activity is a source of oxidative stress and increased superoxide in CACs from inactive subjects that were cultured in the CFU-Hill assay [29]. Increased expression of NADPH oxidase in cultured EPCs from subjects with type 2 diabetes blunts re-endothelialization in a mouse carotid injury model, whereas silencing of NADPH oxidase in these cells reduces superoxide levels and restores re-endothelialization capacity [30]. These findings implicate NADPH oxidase as a factor affecting oxidative stress and CFU-EC number in IGT. Third, hyperglycaemia may also impact CAC function in IGT. High glucose levels impair the invasive capacity of cultured EPCs from healthy subjects [31]. Furthermore, ex vivo physiological hyperglycaemia (12 mmol/L) has been shown to reduce proliferation, migration, and survival of CACs [32]. Our finding that postprandial hyperglycaemia (even at levels below 12 mmol/L) is associated with lower CAC clonogenic potential is concordant with this finding. The lack of association of CFU-EC number with insulin levels seems to implicate hyperglycaemia as opposed to hyperinsulinaemia; however, causal relationships cannot be conclusively established by our data at this time. As we did not detect an association between fasting glucose levels and CFU-EC number, this implies that more prolonged, postprandial hyperglycaemia may be more influential on CAC function than the relatively narrow range of fasting levels in our NGT and IGT subjects (all <7 mmol/L). The specific mechanisms underlying the effects of hyperglycaemia on CAC function and clonogenic potential are not yet established; however, there is some evidence that the effect of high glucose may be mediated by nitric oxide-dependent mechanisms [32,33].
Although most of the aforementioned studies have used cells described as EPCs, the definition of EPCs is variable across studies, and there is evidence that other circulating cells have angiogenic potential. Thus, we report on a broader population of CACs including cell types that (a) promote vascular repair by secretion of pro-angiogenic growth factors at sites of endothelial injury and (b) have robust proliferative capacity and may contribute more directly to neovascularization [34–36]. Our results using the CFU-Hill assay are concordant with published observations in EPCs, adding that the abnormality in CAC function may occur before the development of overt type 2 diabetes and is associated with lower skeletal muscle capillarization in IGT.
Although we report the novel finding of low CFU-EC number in older adults at high risk for type 2 diabetes and associated cardiovascular complications, the strengths and limitations of this study require discussion. First, we studied a continuum of glucose tolerance and groups of IGT and NGT subjects that were well-matched for age, body composition and cardiometabolic risk factors other than glucose intolerance. Subjects with IGT did have lower total cholesterol and LDL-C compared with NGT subjects, but this is likely because half of our IGT subjects were taking statins compared with only one-fourth of our NGT subjects. We included subjects taking statins for hypercholesterolemia because of the ubiquitous use of these medications in older adults at high risk for cardiometabolic diseases. There is evidence that certain statins increase circulating EPC number [37,38], but if statins acted to increase EPC or CAC number in our IGT subjects, we actually may have underestimated the reduction in CFU-EC number in IGT subjects. Although the CFU-Hill assay does generate cells expressing endothelial characteristics [10], conflicting reports exist about the specific identity of cells in the CFU-EC colonies. We acknowledge that the CFU-EC colonies likely contain a mix of pro-angiogenic cell types, but the clonogenic potential of cells in this assay likely represents the pro-angiogenic function of these circulating cells. Finally, this study was conducted to determine whether the clonogenic potential of CACs is lower in older adults with IGT and related to lower skeletal muscle capillarization. We did not measure capillarization in other tissues, nor did we assess macrovascular outcomes such as endothelial vasoreactivity. Future studies will be needed to determine whether associations between CFU-EC number and capillarization are tissue specific and whether CFU-EC number is associated with macrovascular outcomes in IGT.
Conclusion
Our data suggest a depressed state of angiogenesis in IGT, which may negatively affect skeletal muscle capillarization and therefore contribute to worsening glucose homeostasis by limiting insulin and glucose supply to muscle. We consider our findings preliminary evidence that impairments in CAC function may mediate early microvascular changes in the development and progression of IGT to type 2 diabetes. Future studies will be needed to elucidate the mechanisms underlying CAC dysfunction in IGT and to determine whether strategies to improve CAC function can restore normal angiogenesis and skeletal muscle capillarization in older adults with IGT. Because transcapillary transport of insulin is an important determinant of glucose uptake in skeletal muscle, finding strategies to improve CAC function and skeletal muscle capillarization may attenuate further impairments in glucose metabolism in IGT and progression to type 2 diabetes.
Acknowledgments
Our appreciation is extended to the subjects who participated in this study, to our research staff, and to Andrew P. Goldberg, MD, of the University of Maryland School of Medicine. Dr. Prior was supported by a VA Career Development Award (CDA-2-039), and Dr. Ryan is supported by a VA Research Career Scientist Award. This research was supported by a VA Career Development Award (S. J. P.), a VA Merit Review Award (A. S. R.), the Baltimore Veterans Affairs Medical Center Geriatric Research, Education and Clinical Center (GRECC), and the University of Maryland Claude D. Pepper Center (P30-AG028747).
Footnotes
Conflict of interest The authors have no conflict to disclose.
References
- 1.Muntner P, Wildman RP, Reynolds K, DeSalvo KB, Chen J, Fonseca V. Relationship between HbA1c level and peripheral arterial disease. Diabetes Care. 2005;28(8):1981–1987. doi: 10.2337/diacare.28.8.1981. [DOI] [PubMed] [Google Scholar]
- 2.Prior SJ, McKenzie MJ, Joseph LJ, et al. Reduced skeletal muscle capillarization and glucose intolerance. Microcirculation. 2009;16(3):203–212. doi: 10.1080/10739680802502423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudbjornsdottir S, Sjostrand M, Strindberg L, Wahren J, Lonnroth P. Direct measurements of the permeability surface area for insulin and glucose in human skeletal muscle. J Clin Endocrinol Metab. 2003;88(10):4559–4564. doi: 10.1210/jc.2003-030434. [DOI] [PubMed] [Google Scholar]
- 4.Ader M, Bergman RN. Importance of transcapillary insulin transport to dynamics of insulin action after intravenous glucose. Am J Physiol. 1994;266(1 Pt 1):E17–E25. doi: 10.1152/ajpendo.1994.266.1.E17. [DOI] [PubMed] [Google Scholar]
- 5.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Miorin M, Facco M, et al. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45(9):1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 7.Fadini GP, Pucci L, Vanacore R, et al. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia. 2007;50(10):2156–2163. doi: 10.1007/s00125-007-0732-y. [DOI] [PubMed] [Google Scholar]
- 8.Tepper OM, Galiano RD, Capla JM, et al. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106(22):2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 9.Thum T, Fraccarollo D, Schultheiss M, et al. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56(3):666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 10.Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2012;35(Suppl 1):S64–S71. doi: 10.2337/dc12-s064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 13.Hennessey JV, Chromiak JA, Della VS, Guertin J, MacLean DB. Increase in percutaneous muscle biopsy yield with a suction-enhancement technique. J Appl Physiol. 1997;82(6):1739–1742. doi: 10.1152/jappl.1997.82.6.1739. [DOI] [PubMed] [Google Scholar]
- 14.Porter MM, Stuart S, Boij M, Lexell J. Capillary supply of the tibialis anterior muscle in young, healthy, and moderately active men and women. J Appl Physiol. 2002;92(4):1451–1457. doi: 10.1152/japplphysiol.00744.2001. [DOI] [PubMed] [Google Scholar]
- 15.Porter MM, Koolage CW, Lexell J. Biopsy sampling requirements for the estimation of muscle capillarization. Muscle Nerve. 2002;26(4):546–548. doi: 10.1002/mus.10221. [DOI] [PubMed] [Google Scholar]
- 16.Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese post-menopausal women. J Gerontol A Biol Sci Med Sci. 2011;66(9):1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prior SJ, Joseph LJ, Brandauer J, Katzel LI, Hagberg JM, Ryan AS. Reduction in midthigh low-density muscle with aerobic exercise training and weight loss impacts glucose tolerance in older men. J Clin Endocrinol Metab. 2007;92(3):880–886. doi: 10.1210/jc.2006-2113. [DOI] [PubMed] [Google Scholar]
- 18.Caballero AE, Arora S, Saouaf R, et al. Microvascular and macrovascular reactivity is reduced in subjects at risk for type 2 diabetes. Diabetes. 1999;48(9):1856–1862. doi: 10.2337/diabetes.48.9.1856. [DOI] [PubMed] [Google Scholar]
- 19.Li M, Ho JC, Lai KW, et al. The decrement in circulating endothelial progenitor cells (EPCs) in type 2 diabetes is independent of the severity of the hypoadiponectemia. Diabetes Metab Res Rev. 2011;27(2):185–194. doi: 10.1002/dmrr.1159. [DOI] [PubMed] [Google Scholar]
- 20.Cubbon RM, Kahn MB, Wheatcroft SB. Effects of insulin resistance on endothelial progenitor cells and vascular repair. Clin Sci (Lond) 2009;117(5):173–190. doi: 10.1042/CS20080263. [DOI] [PubMed] [Google Scholar]
- 21.Hashimoto K, Kasayama S, Yamamoto H, Kurebayashi S, Kawase I, Koga M. Strong association of C-reactive protein with body mass index and 2-h post-challenge glucose in non-diabetic, non-smoker subjects without hypertension. Diabet Med. 2004;21(6):581–585. doi: 10.1111/j.1464-5491.2004.01212.x. [DOI] [PubMed] [Google Scholar]
- 22.Straczkowski M, Kowalska I, Dzienis-Straczkowska S, et al. Changes in tumor necrosis factor-alpha system and insulin sensitivity during an exercise training program in obese women with normal and impaired glucose tolerance. Eur J Endocrinol. 2001;145(3):273–280. doi: 10.1530/eje.0.1450273. [DOI] [PubMed] [Google Scholar]
- 23.Verma S, Kuliszewski MA, Li SH, et al. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109(17):2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 24.Seeger FH, Haendeler J, Walter DH, et al. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111(9):1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 25.Vijayalingam S, Parthiban A, Shanmugasundaram KR, Mohan V. Abnormal antioxidant status in impaired glucose tolerance and non-insulin-dependent diabetes mellitus. Diabet Med. 1996;13(8):715–719. doi: 10.1002/(SICI)1096-9136(199608)13:8<715::AID-DIA172>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 26.Urakawa H, Katsuki A, Sumida Y, et al. Oxidative stress is associated with adiposity and insulin resistance in men. J Clin Endocrinol Metab. 2003;88(10):4673–4676. doi: 10.1210/jc.2003-030202. [DOI] [PubMed] [Google Scholar]
- 27.Awad O, Jiao C, Ma N, Dunnwald M, Schatteman GC. Obese diabetic mouse environment differentially affects primitive and monocytic endothelial cell progenitors. Stem Cells. 2005;23(4):575–583. doi: 10.1634/stemcells.2004-0185. [DOI] [PubMed] [Google Scholar]
- 28.Fleissner F, Thum T. Critical role of the nitric oxide/reactive oxygen species balance in endothelial progenitor dysfunction. Antioxid Redox Signal. 2011;15(4):933–948. doi: 10.1089/ars.2010.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jenkins NT, Witkowski S, Spangenburg EE, Hagberg JM. Effects of acute and chronic endurance exercise on intracellular nitric oxide in putative endothelial progenitor cells: role of NAPDH oxidase. Am J Physiol Heart Circ Physiol. 2009;297(5):H1798–H1805. doi: 10.1152/ajpheart.00347.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorrentino SA, Bahlmann FH, Besler C, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116(2):163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 31.Urbich C, Dernbach E, Rossig L, Zeiher AM, Dimmeler S. High glucose reduces cathepsin L activity and impairs invasion of circulating progenitor cells. J Mol Cell Cardiol. 2008;45(3):429–436. doi: 10.1016/j.yjmcc.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Krankel N, Adams V, Linke A, et al. Hyperglycemia reduces survival and impairs function of circulating blood-derived progenitor cells. Arterioscler Thromb Vasc Biol. 2005;25(4):698–703. doi: 10.1161/01.ATV.0000156401.04325.8f. [DOI] [PubMed] [Google Scholar]
- 33.Chen YH, Lin SJ, Lin FY, et al. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56(6):1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 34.Gulati R, Jevremovic D, Peterson TE, et al. Diverse origin and function of cells with endothelial phenotype obtained from adult human blood. Circ Res. 2003;93(11):1023–1025. doi: 10.1161/01.RES.0000105569.77539.21. [DOI] [PubMed] [Google Scholar]
- 35.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Medina RJ, O'Neill CL, Sweeney M, et al. Molecular analysis of endothelial progenitor cell (EPC) subtypes reveals two distinct cell populations with different identities. BMC Med Genomics. 2010;3:18. doi: 10.1186/1755-8794-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter DH, Rittig K, Bahlmann FH, et al. Statin therapy accelerates reendothelialization: a novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation. 2002;105(25):3017–3024. doi: 10.1161/01.cir.0000018166.84319.55. [DOI] [PubMed] [Google Scholar]
- 38.Dimmeler S, Aicher A, Vasa M, et al. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108(3):391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]


