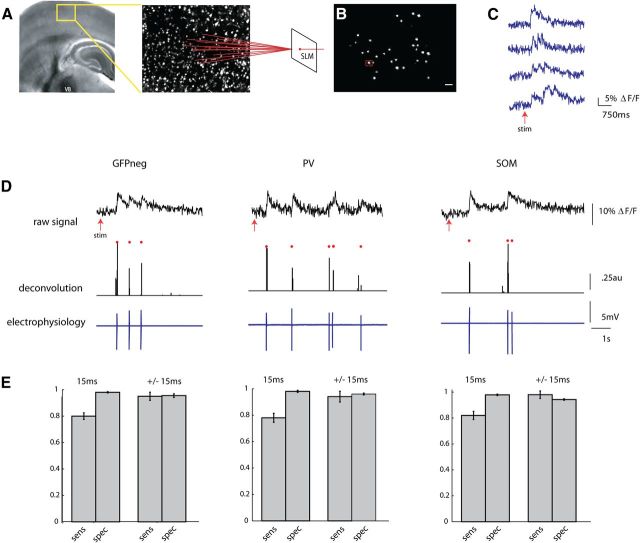
Figure 2.
Two-photon fast calcium imaging with a single-spike deconvolution algorithm. A, Light micrograph of a S1 thalamocortical slice preparation with intact thalamic input nucleus VB, thalamocortical axons, and the somatosensory cortex. A stimulating electrode is placed in VB. Superimposed yellow box indicates location, over layers 2/3 and 4, of illustrated two photon z stack to right. Neurons pictured in this field are loaded with fura-2 AM dye, and targeted with an SLM (far right). B, Two-photon image of a single frame showing neuronal cell bodies targeted with two photon illumination with the SLM. Cell outlined in red was targeted in cell-attached mode (D). Scale bar, 30 μm. C, Examples of fluorescence signals showing changes in fluorescence, normalized to baseline (ΔF/F), from four cells imaged at 66.6 Hz with an EMCCD. D, Three examples from calibration experiments in which a PC (left), a PV cell (middle), and SOM cell (right) were targeted for cell attached recording during simultaneous stimulation of the thalamus. Top trace shows raw fluorescence signal from that cell imaged at 66.6 Hz. Middle trace is the deconvolution of the calcium signals using parameters obtained from electrophysiology to obtain estimated spike times. Red dots above both traces indicate the time of the actual spikes. Bottom trace shows the associated electrophysiological trace. E, Sensitivity (true-positive rate) and specificity (1-false-positive rate) of the deconvolution algorithm. These rates were calculated while allowing for either no window around each spike to search for a signal (15 ms), or for a window of ± 1 frame around each spike (±15 ms).