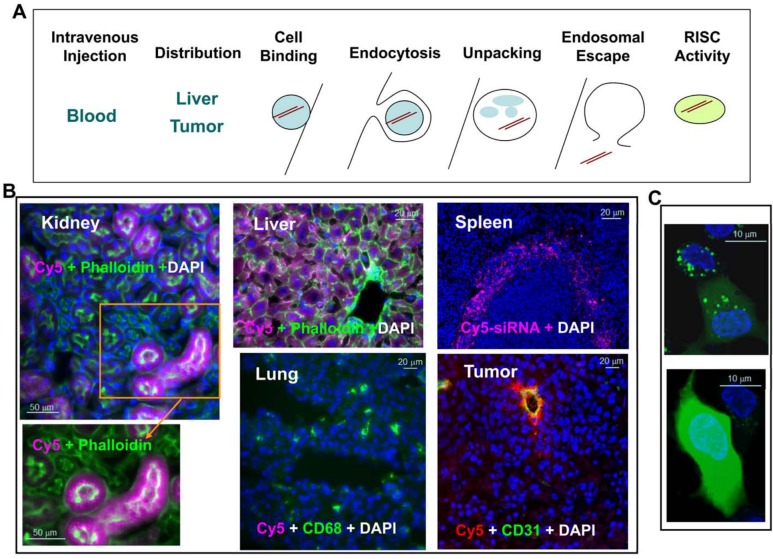
Figure 2.
Barriers to lipid nanoparticle (LNP)–mediated small interfering RNA (siRNA) delivery and assay development. (A) Delivery is the key challenge to be addressed before an siRNA therapeutic can be fully realized. Successful delivery must enable bypass of multiple biological barriers, including blocking blood nuclease digestion of siRNA by chemical modification of the siRNA duplex, improving target-specific tissue biodistribution and cellular uptake, increasing cell binding via electrostatic interaction of the LNP and cell membrane or ligand and cell surface receptor, enhancing receptor-mediated endocytosis, increasing the efficiency of unpacking siRNA from the lipid nanoparticle delivery vehicle, enhancing the endosomal escape of siRNA to the cytosol, and improving siRNA loading into the RNA-induced silencing complex (RISC) in the cytosol for target mRNA cleavage (courtesy of Matt Stanton and Steve Colletti). (B) Tissue distribution of LNP-siRNA-Cy5 in liver, spleen, lung, kidney, and KB3 (a nasopharyngeal carcinoma cell line) tumor xenograft. LNP-siRNA labeled with Cy5 was intravenously injected into a mouse at 3 mg/kg. Different tissues were collected 2 hr postdosing and cryosections were analyzed by epifluorescence microscopy. All images were taken at ×20 magnification. siRNA-Cy5 (purple) mainly distributed to the liver, the red pulp of the spleen, and the proximal tubules of the kidney but not much to the lung. Phalloidin (green) outlines cell membrane and CD68 (green) stains macrophages. In the tumor section, siRNA-Cy5 (red) was mainly located at CD31-stained vessels and adjacent KB3 tumor cells but did not penetrate very far. (C) Dextran endosomal escape assay measuring the endosomal release of dextran (green) from punctate endosomes into diffused signals in the cytosol in HepG2 cells in vitro (with permission from Eileen Walsh and Bonnie Howell, unpublished data). Bars = 50 µm (B: kidney); 20 µm (B: liver and lung); 20 µm (B: spleen and tumor); 10 µm (C).