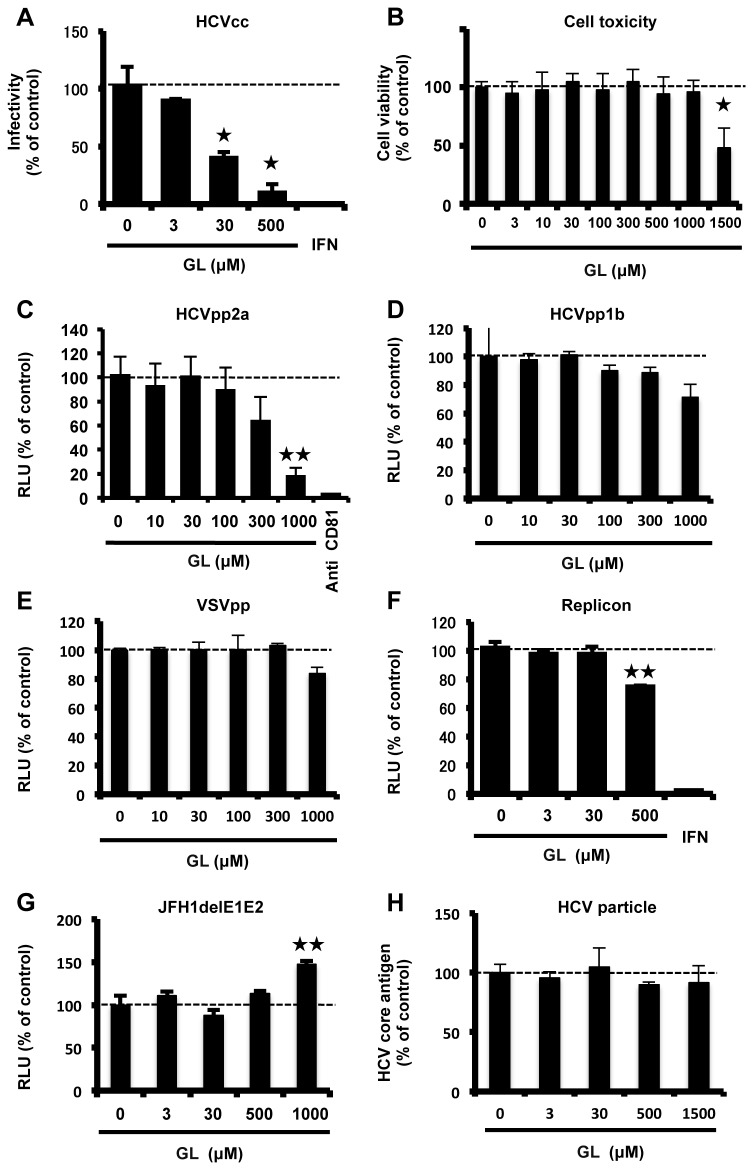
Figure 1. Anti-HCV effects of GL.
(A) HCVcc-infected cells were treated with various concentrations of GL for 72 hours. Naïve Huh7 cells were inoculated with supernatant and cultured for 72 hours. Infectivity was determined by immunostaining. (B) Cell viability was assessed using Cell Titer-Glo Luminescent Cell Viability Assay. Huh7 cells were infected with HCVpp2a (C), HCVpp1b (D), and VSVpp (E) in various concentrations of GL for 24 hours, and then medium was replaced. Effects of GL on entry of HCVpp and VSVpp were determined by measuring the luciferase activity at 72 hours post-transfection. (F) Huh7 cells harboring the type-2a subgenomic replicon were treated with various concentrations of GL for 72 hours. Replication efficiency of the replicon was estimated by measuring the luciferase activity. (G) The effects of GL on HCV replication were tested by electroporation of HCV RNA lacking E1E2 (JFH1delE1E2). (H) HCV particles were treated with increasing concentrations (0 to 1500µM) of GL. The viral samples were then used to inoculate Huh7 cells with GL-containing medium. Several hours post-infection, medium was replaced with DMEM without GL. The levels of HCV core antigen of the medium were determined at 72 h postinfection (p.i.). IFN (300 IU/ml) was used as a positive control for reduced HCV replication. Anti-human CD81 antibody (10 µg/ml) was used as a positive control for reduced HCV entry to the cells. Results are expressed as the mean ± SD of the percent of the control from four independent experiments. *P < 0.05, **P < 0.005 versus control (0 µM treatment).