Abstract
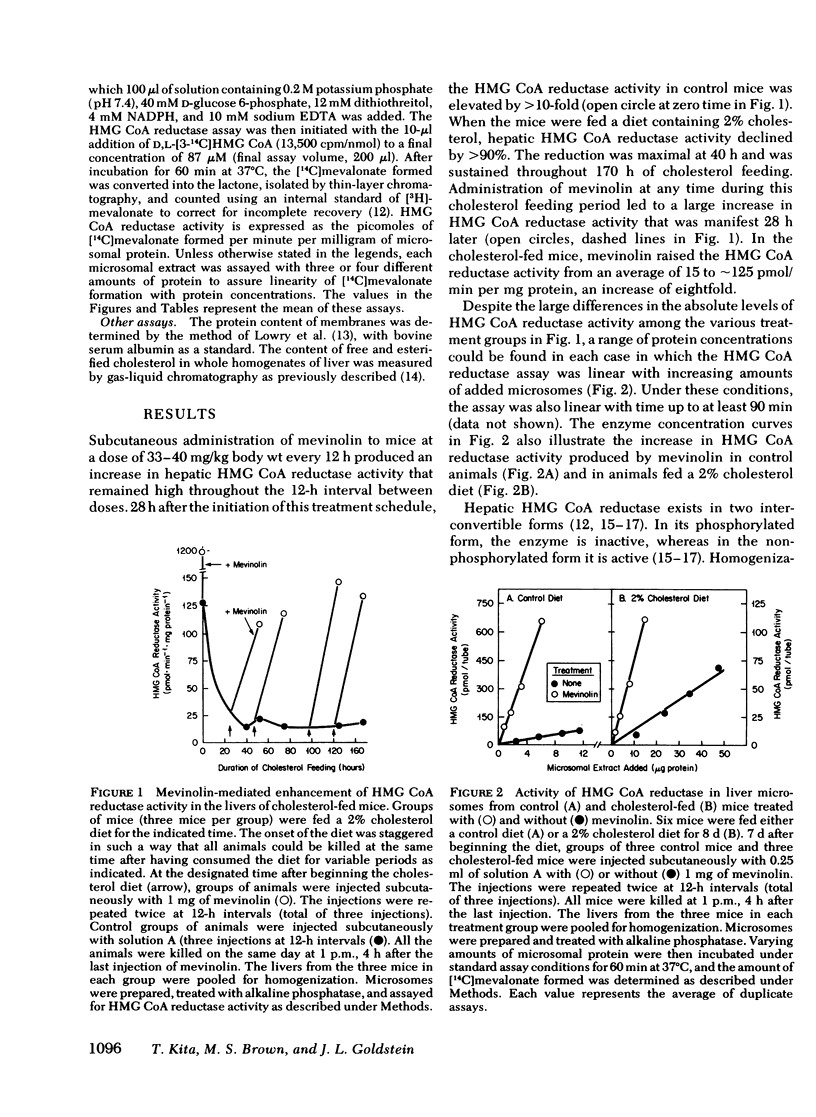
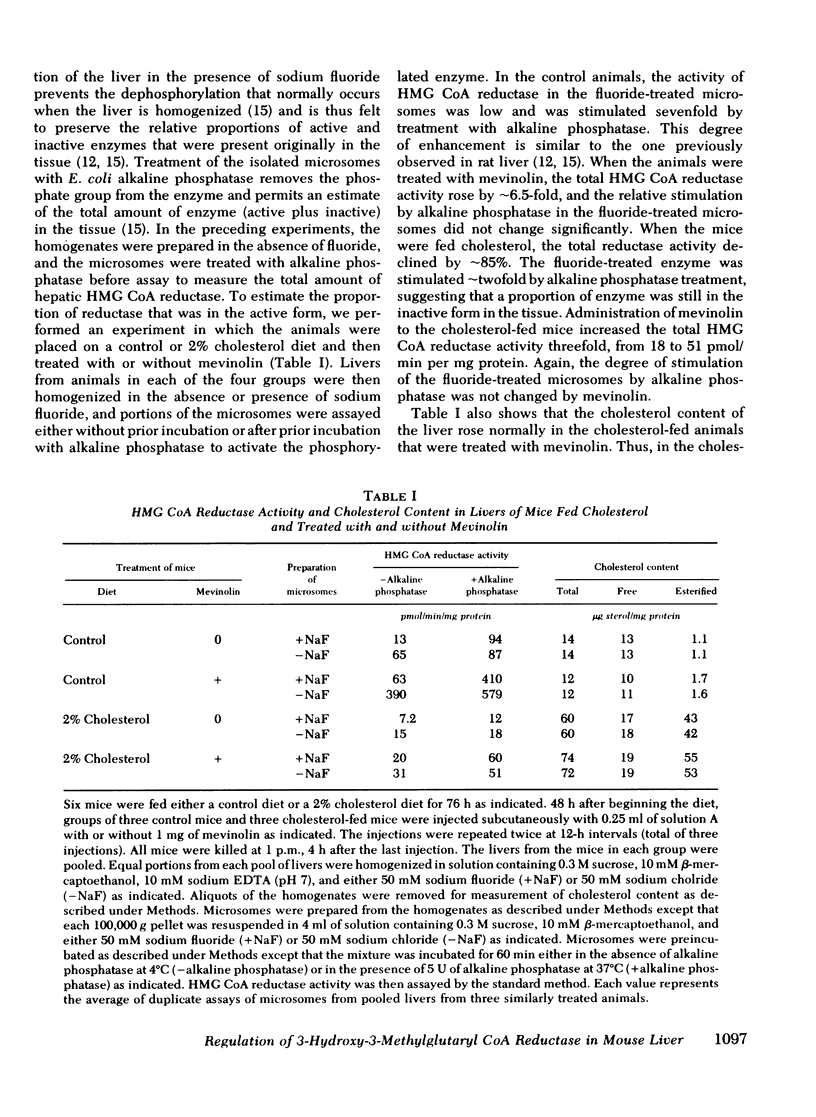
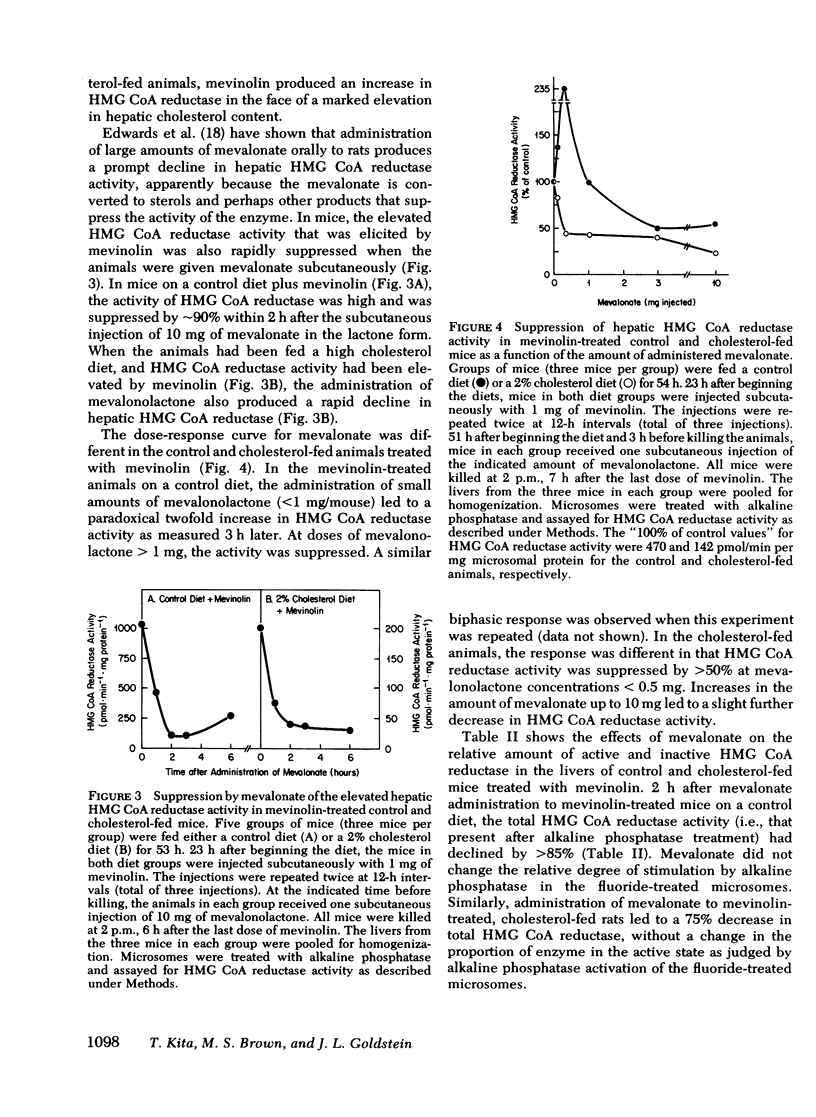
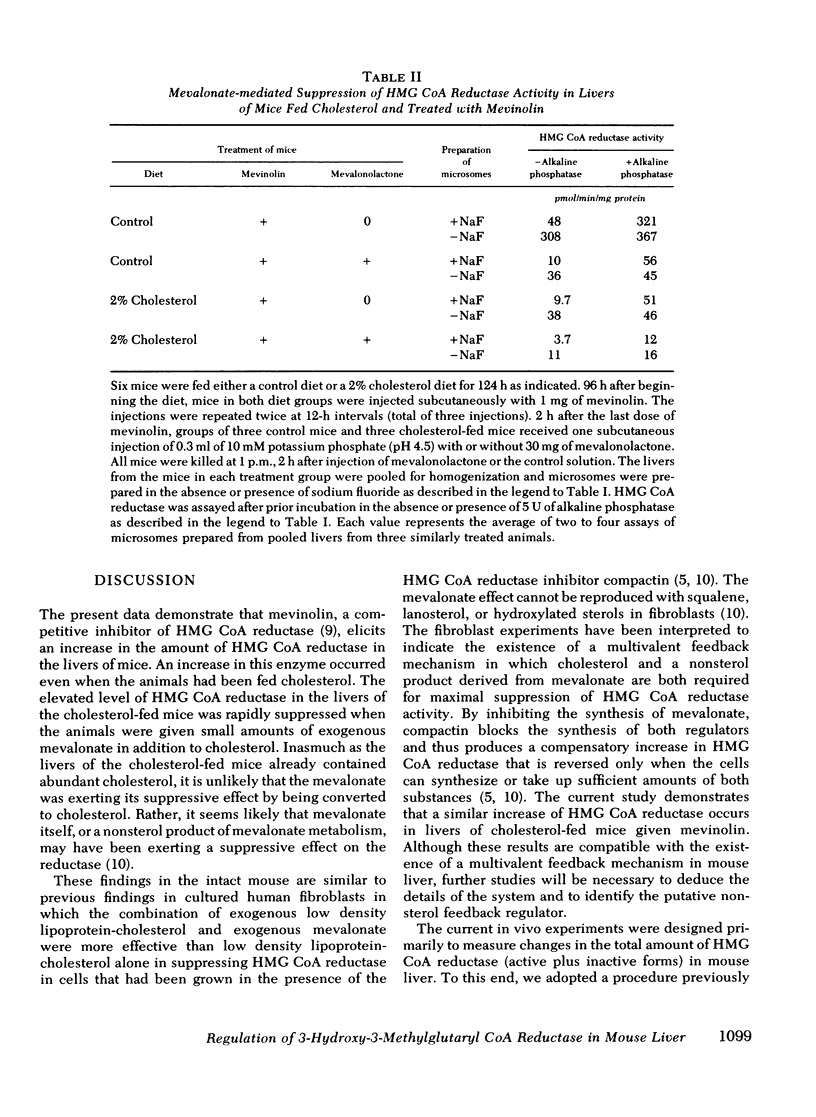
Compactin (ML-236B) and the related compound, mevinolin, are competitive inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMG CoA reductase), the rate-controlling enzyme in cholesterol synthesis. Previous studies have shown that administration of compactin to cultured cells elicits a compensatory increase in the amount of HMG CoA reductase in the cells. A similar increase in HMG CoA reductase has been reported in livers of rats and mice that have been treated with compactin. In this study, we explore the mechanism for the mevinolin-mediated increase in hepatic HMG CoA reductase in mice that have been fed a control diet and a 2% cholesterol diet. Administration of mevinolin to mice on a control diet produced a 6- to 10-fold increase in the amount of HMG CoA reductase in liver microsomes. When mice were fed the cholesterol-enriched diet, cholesterol accumulated in the liver and HMG CoA reductase declined by 90%. The administration of mevinolin to cholesterol-fed mice produced a three to eightfold increase in HMG CoA reductase. Despite the abundant amount of cholesterol that was already present in the livers of the mevinolin-treated, cholesterol-fed animals, their elevated HMG CoA reductase could be rapidly suppressed by the subcutaneous injection of small amounts of mevalonate, the product of HMG CoA reductase. These data are compatible with the existence in mouse liver of a multivalent feedback regulatory mechanism for HMG CoA reductase in which suppression of the enzyme requires both a sterol and a nonsterol substance derived from mevalonate. By blocking mevalonate synthesis, mevinolin activates this regulatory mechanism, and this in turn causes an increase in hepatic HMG CoA reductase. The ability to suppress the elevated HMG CoA reductase with mevalonate may prove useful in potentiating the effectiveness of mevinolin as a hypocholesterolemic agent.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts A. W., Chen J., Kuron G., Hunt V., Huff J., Hoffman C., Rothrock J., Lopez M., Joshua H., Harris E. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg Z. H., Stonik J. A., Brewer H. B., Jr 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3678–3682. doi: 10.1073/pnas.75.8.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. G., Smale T. C., King T. J., Hasenkamp R., Thompson R. H. Crystal and molecular structure of compactin, a new antifungal metabolite from Penicillium brevicompactum. J Chem Soc Perkin 1. 1976;(11):1165–1170. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L., Kaneko I., Endo A. Induction of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts incubated with compactin (ML-236B), a competitive inhibitor of the reductase. J Biol Chem. 1978 Feb 25;253(4):1121–1128. [PubMed] [Google Scholar]
- Brown M. S., Faust J. R., Goldstein J. L. Role of the low density lipoprotein receptor in regulating the content of free and esterified cholesterol in human fibroblasts. J Clin Invest. 1975 Apr;55(4):783–793. doi: 10.1172/JCI107989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Dietschy J. M. Active and inactive forms of 3-hydroxy-3-methylglutaryl coenzyme A reductase in the liver of the rat. Comparison with the rate of cholesterol synthesis in different physiological states. J Biol Chem. 1979 Jun 25;254(12):5144–5149. [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Multivalent feedback regulation of HMG CoA reductase, a control mechanism coordinating isoprenoid synthesis and cell growth. J Lipid Res. 1980 Jul;21(5):505–517. [PubMed] [Google Scholar]
- Edwards P. A., Popják G., Fogelman A. M., Edmond J. Control of 3-hydroxy-3-methylglutaryl coenzyme A reductase by endogenously synthesized sterols in vitro and in vivo. J Biol Chem. 1977 Feb 10;252(3):1057–1063. [PubMed] [Google Scholar]
- Endo A., Kuroda M., Tanzawa K. Competitive inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase by ML-236A and ML-236B fungal metabolites, having hypocholesterolemic activity. FEBS Lett. 1976 Dec 31;72(2):323–326. doi: 10.1016/0014-5793(76)80996-9. [DOI] [PubMed] [Google Scholar]
- Endo A. Monacolin K, a new hypocholesterolemic agent produced by a Monascus species. J Antibiot (Tokyo) 1979 Aug;32(8):852–854. doi: 10.7164/antibiotics.32.852. [DOI] [PubMed] [Google Scholar]
- Endo A., Tsujita Y., Kuroda M., Tanzawa K. Effects of ML-236B on cholesterol metabolism in mice and rats: lack of hypocholesterolemic activity in normal animals. Biochim Biophys Acta. 1979 Nov 21;575(2):266–276. [PubMed] [Google Scholar]
- Gibson D. M., Ingebritsen T. S. Reversible modulation of liver hydroxymethylglutaryl CoA reductase. Life Sci. 1978 Dec 31;23(27-28):2649–2664. doi: 10.1016/0024-3205(78)90644-6. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Helgeson J. A., Brown M. S. Inhibition of cholesterol synthesis with compactin renders growth of cultured cells dependent on the low density lipoprotein receptor. J Biol Chem. 1979 Jun 25;254(12):5403–5409. [PubMed] [Google Scholar]
- Kaneko I., Hazama-Shimada Y., Endo A. Inhibitory effects on lipid metabolism in cultured cells of ML-236B, a potent inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem. 1978 Jun 15;87(2):313–321. doi: 10.1111/j.1432-1033.1978.tb12380.x. [DOI] [PubMed] [Google Scholar]
- Kuroda M., Tsujita Y., Tanzawa K., Endo A. Hypolipidemic effects in monkeys of ML-236B, a competitive inhibitor of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Lipids. 1979 Jun;14(6):585–589. doi: 10.1007/BF02533537. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nordstrom J. L., Rodwell V. W., Mitschelen J. J. Interconversion of active and inactive forms of rat liver hydroxymethylglutaryl-CoA reductase. J Biol Chem. 1977 Dec 25;252(24):8924–8934. [PubMed] [Google Scholar]
- Yamamoto A., Sudo H., Endo A. Therapeutic effects of ML-236B in primary hypercholesterolemia. Atherosclerosis. 1980 Mar;35(3):259–266. doi: 10.1016/0021-9150(80)90124-0. [DOI] [PubMed] [Google Scholar]


