Abstract
Context
Encouraging use of hospice and minimizing the use of cure-oriented, aggressive interventions that detract from quality of life in the last month of life are specific targets for improvement in cancer care.
Objectives
To evaluate the effect of an interdisciplinary Cancer Support Team (CST) on quality of care and quality of life in patients with advanced cancers.
Methods
A non-randomized clinical trial was conducted, comparing outcomes before and after the integration of an interdisciplinary CST in routine care of adults with Stage III or IV lung, gastrointestinal or gynecologic cancer. In the control arm, patients (n=332) received usual care; following initiation of the intervention arm, eligible patients (n=278) received the CST intervention. The intervention consisted of individualized care coordination, symptom management, education, psychosocial and spiritual support, and advance care planning throughout the 15-month study period. Quality of end-of-life care was measured through an “aggressiveness of care” index. Health-related quality of life (HRQOL) was measured with the Functional Assessment of Cancer Therapy–General (FACT-G).
Results
There were no statistically significant differences between groups on specific indicators of quality of care. Surviving subjects with higher survival expectancy (who also reported better baseline quality of life) in the intervention arm had the greatest improvement in HRQOL scores, compared with the other three groupings of survival expectancy by treatment group (high vs. low by intervention vs. control) (P=0.044).
Conclusion
Individually tailored supportive services from an interdisciplinary team are associated with improved HRQOL in some patients. This has implications for the potential benefits that can be accrued from providing intensive support to all patients, even those who may appear to be at less risk for distress. There also are important methodological considerations in using aggressiveness of care indices as a measure of quality of care.
Keywords: supportive cancer care, palliative care in cancer, aggressiveness of care index
Introduction
Cancer remains the second leading cause of death in the U.S., with more than half a million deaths having been projected for 2012 (1). Because of its prevalence, mortality rates, and frequent need for expert symptom management, cancer has been the focus of efforts to institute systematic changes in end-of-life care (2,3). Recommendations for improving the quality of care have been operationalized by the National Quality Forum (NQF). The NQF is a nonprofit organization, comprising a variety of health care stakeholders in the U.S.; its mission is to build consensus on national priorities and goals for performance improvement, in part through developing and endorsing national consensus standards for measuring and publicly reporting on performance. In 1999, the NQF published consensus standards for quality end-of-life care for cancer patients (http://www.qualityforum.org) that can be used to assess opportunities for improvement in care through assuring access to hospice and limiting the use of aggressive, cure-oriented interventions at the end of life. These standards have been used to describe trends and evaluate systems of care and include such items as the proportion of patients who received chemotherapy in the last 14 days of life, or had more than one hospitalization in the last 30 days, and the percent of patients admitted to hospice (4-7).
Integrated, coordinated models of care have been recommended in order to assure that patients have access to curative therapies as well as management of physical, psychological, and spiritual needs. Although there have been some encouraging reports of the effectiveness of early palliative medicine consultation with specific cancer patient populations (8) and focused psychoeducational support programs (9), there have been few controlled trials of fully integrated and coordinated services within comprehensive cancer centers. In part, this reflects the operational difficulties of instituting major structural changes in the care delivery system, and also the challenges of conducting rigorous tests of changes under real-world conditions.
This report describes a trial of integrating an interdisciplinary Cancer Support Team (CST), composed of advanced practice nurses (APNs), social workers (SWs), and a spiritual care counselor (SCC), as part of the routine care delivery system for patients with a variety of cancer types. Extending the findings of other trials of early palliative or supportive care programs, the CST was an interdisciplinary team, designed to address both the physical (i.e., symptom) issues as well as social and spiritual concerns. The primary aim was to measure the effect of the CST, using the established NQF quality of end-of-life care indicators, on health-related quality of life (HRQOL) in a population with advanced cancer. The primary outcome was the quality of end-of-life care, including hospice use and aggressiveness of care indicators. We report on the outcomes of the trial and the implications both for cancer care delivery systems and future evaluations of palliative care programs.
Methods
Study Population
All adult patients with newly diagnosed Stage III or IV lung, gastrointestinal (GI), or gynecologic (GYN) cancer, admitted to the outpatient clinic of a comprehensive cancer center were screened for eligibility. In addition to age (18 years or older) and cancer type, eligibility criteria included Eastern Cooperative Oncology Group (ECOG) performance status ≤ 3, capacity to provide informed consent, and intention to receive treatment at the cancer center.
A quasi-experimental design was used to measure the quality of care and quality of life outcomes associated with integration of an interdisciplinary supportive care team in routine care. With this design, subjects who were receiving “usual care” were enrolled, allocated to the control arm, and data collection begun. Once the target control arm sample had been accrued, the intervention was initiated and subsequent eligible subjects were allocated to the experimental arm. This design was chosen because of the very high likelihood of significant contamination if patients who were receiving care from the same providers in the same setting were concurrently randomized to the two groups.
In the control period, patients received usual access to cancer center APNs, SWs, and the SCC, depending on the assessment of need by the primary oncology team. Patients did not receive any routine ancillary services unless referred by a physician or nurse. Services were not protocol-driven and were provided independently by each person, rather than as a formal coordinated team. Once the CST intervention was implemented, all patients meeting eligibility criteria received the intervention as part of routine care.
The study was performed at Case Western Reserve University and University Hospitals Case Medical Center, Seidman Cancer Center, Cleveland, OH. The study was approved by the study site's institutional review board and registered on ClinicalTrials.gov (NCT 00684801).
Intervention
Patients in the intervention group received support and care coordination provided by the interdisciplinary CST. All members of the CST were experienced oncology professionals. Prior to initiating the intervention and throughout the first six months, weekly team meetings were held to review and discuss the protocol and address questions as they arose. The program consisted of eight components: 1) baseline assessment of symptoms, distress, and social and spiritual concerns of the patient and family; 2) summary of a plan for supportive services was entered in the medical record; 3) ongoing provision of symptom management, education, and psychosocial and spiritual support according to patient need; 4) introduction of advance care planning discussions as early as possible; 5) a minimum of monthly contacts with the patient in the clinic during treatment or by phone if the patient had no clinic appointments, for the duration of the 15-month study period; 6) daily availability of team members to the patient and family by phone for questions; 7) regular (monthly or quarterly) meetings with each oncologist to review and coordinate care plans for his/her patients; and 8) referral to and coordination with community providers (home care, palliative care, or hospice) when appropriate.
A member of the CST met each patient by the second clinic appointment, explained the purpose of the team (to provide supportive services as an adjunct to his/her cancer treatment), obtained a detailed history and performed the baseline assessment. Patients and family caregivers had access to all members of the CST, but the focus and amount of services (e.g., help with financial concerns, intensive symptom management, spiritual counseling, etc.) was determined by patient and caregiver need. Patients were re-assessed frequently during active treatment and encouraged to contact team members at any time with questions or needs. The team reviewed all new patients after admission and met weekly to review patients and coordinate plans of care.
Procedures and Measures
Following informed consent, the research assistant (RA) interviewed the patient and caregiver to obtain demographic and clinical information as well as HRQOL data. Given the allocation procedure used, it was not possible for the RA to be blinded to group assignment. Telephone or in-clinic interviews were conducted again three, nine, and 15 months later. A monthly phone call was made by the RA to determine patient status (survival and location) and resource use (initiation of home care, emergency room visits, or initiation of hospice). The medical record was reviewed at study end (death, completion of the 15-month study period, or initiation of hospice) for data related to the NQF consensus standards.
Demographic data were obtained through in-person interview and clinical data were obtained from medical record review. Applying the NQF standards, we computed an “aggressiveness of care” index (4-6), comprising six items: administration of intravenous (IV) chemotherapy in the last 14 days of life; within the last 30 days of life, the administration of new IV or oral chemotherapy; more than one emergency department visit; more than one hospitalization; an intensive care unit (ICU) admission; and less than three days of hospice (4, 5). Quality of life was measured with the Functional Assessment of Cancer Therapy–General (FACT-G) (10).
Statistical Analysis
Using the quality outcome of most interest (percent of patients who died who had transitioned to hospice), power analysis indicated a total sample of at least 550 was needed. This allowed detection of a small-to-moderate effect size (f=0.13) with a power of 0.85 and α = 0.05.
Frequencies and measures of central tendency were used to describe the sample. For group comparisons, one-way analysis of variance (ANOVA) tests for continuous variables and χ2 for categorical variables were employed.
Multivariate analyses were used to examine the impact of group upon key outcome variables, controlling for identified group differences (Table 1). Most outcome variables were strongly related to cancer type and stage. Given this, we developed an index to reflect mortality risk (or, conversely, survival expectation) by using five-year Surveillance Epidemiology and End Results (SEER) mortality data to assign survival expectation to each patient according to cancer type and stage (www.cancer.gov). Higher numbers for this variable reflected a higher probability of five-year survival. This variable was used as a covariate in all multivariate analyses.
Table 1.
Comparison of Demographic and Clinical Variables Between Experimental and Control Patients (N=610)
| Variable | Control (n=332) | Experimental (n=278) | F | P |
|---|---|---|---|---|
| Mean (SD) 95% CI | Mean (SD) 95% CI | |||
| Age (patient) | 62.93 (11.35) 61.70 – 64.15 | 62.67 (11.33) 61.33 – 64.00 | 0.78 | 0.78 |
| Charlson Comorbidity Index | 0.61 ( 1.18) 0.48 – 0.74 | 0.87 ( 1.31) 0.71 – 1.02 | 6.29 | 0.01 |
| n (%) | n (%) | χ 2 | P | |
|---|---|---|---|---|
| Female Gender | 218 (65.7) | 159 (57.2) | 4.59 | 0.03 |
| Caucasian Race | 273 (82.2) | 202 (72.7) | 10.22 | 0.04 |
| Married | 218 (65.7) | 158 (56.8) | 4.99 | 0.03 |
| Household Income <$50,000/yr | 155 (50.7) | 149 (58.7) | 3.67 | 0.16 |
| Cancer Type | 34.9 | 0.001 | ||
| GI/Colorectal | 146 (44.0) | 128 (46.0) | ||
| Lung | 82 (24.7) | 114 (41.0) | ||
| GYN | 104 (31.3) | 36 (12.9) | ||
| Cancer Stage | 17.9 | 0.001 | ||
| III | 164 (50.0) | 113 (39.9) | ||
| IV | 164 (50.0) | 167 (60.1) | ||
| Prior Cancer: Yes | 58 (17.6) | 56 (20.1) | 0.7 | 0.42 |
| Clinical Trial: Yes | 52 (16.5) | 40 (14.4) | 0.5 | 0.49 |
| Received Chemotherapy | 77 (53.5) | 242 (87.1) | 57.9 | 0.001 |
| Received Radiation Therapy | 23 (16.0) | 73 (26.3) | 5.7 | 0.02 |
| Received Surgery | 45 (31.2) | 102 (36.7) | 1.2 | 0.30 |
Multiple linear regression was used for continuous outcome variables (hospice days, hospital admission days, number of hospital admissions), and logistic regression was used for the dichotomous outcome variable, hospice referral. There were no multicollinearity or independence of observation concerns and the logistic model assumption of linear relationships between the logit and the continuous covariates was verified for the logistic model. Finally, we used a linear mixed effects modeling approach to examine HRQOL over time. For all analyses, a two-sided P-value < 0.05 was considered to be statistically significant. Linear mixed effects model parameter estimation was performed via restricted maximum likelihood (REML) using PROC MIXED in SAS v. 9.22 (SAS Institute Inc., Cary, NC) (11).
Results
Sample Characteristics
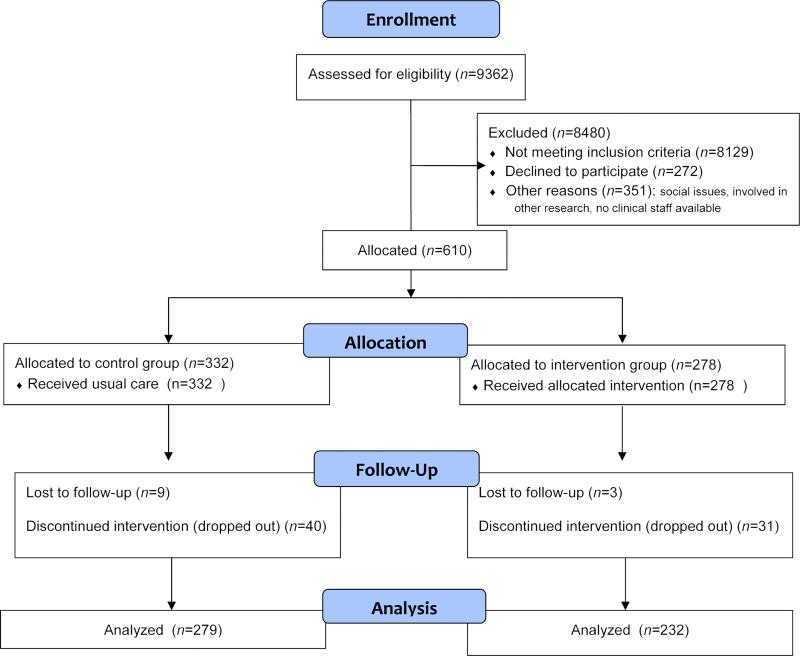
Of the 882 patients approached to participate, 272 (30%) refused. As seen in Fig. 1, 610 subjects were enrolled and consented to take part in the study (control=332 and experimental=278). The average age of patients was 62.8 (11.3) years, with a majority being female (61.8%) and Caucasian (77.9%). More than half had Stage IV cancer (54.6%), with the following cancer types: GI (44.9%), lung (32.1%), and GYN (23.0%). Intervention subjects were more likely to have lung cancer, be Stage IV, have higher comorbidity, and die and were less likely to be female or Caucasian when compared with the control group (Table 1). Because of these significant differences between groups on variables with the potential to affect both HRQOL and use of aggressive interventions at the end of life, our analytic strategy incorporated steps to examine possible confounding effects.
Fig. 1.
Subject flow diagram.
Hospice Use
Logistic regression was used to examine whether group was related to hospice use for patients who died, when controlling for gender, race, and survival expectation. We also conducted multiple regression analyses to examine the relationship between group and hospice days when controlling for the same variables. In both models, group was not statistically significant and the only variable that had a significant relationship to each outcome variable was survival expectation (hospice referral: odds ratio [OR] 1.04, 95% confidence interval [CI] 1.027, 1.05, P=0.0001; hospice days: β=0.15, P=0.03).
End-of-Life Measures: Aggressiveness Indices
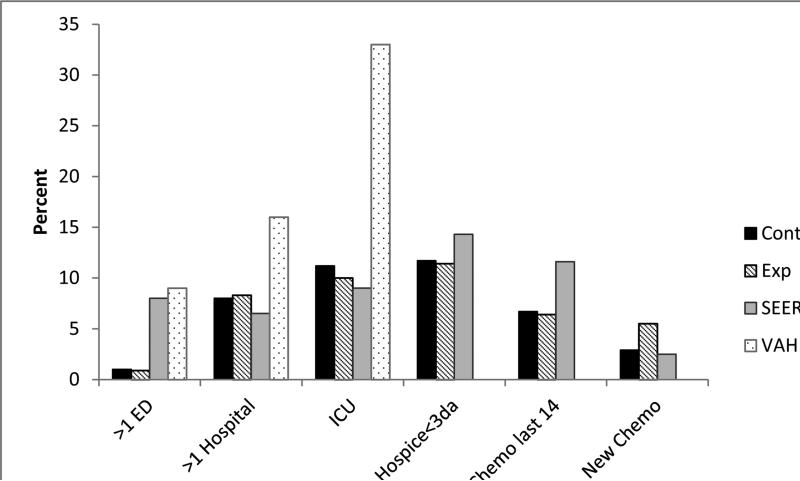
As part of the quality of care analysis, we also examined the use of aggressive end-of-life measures in the last 30 days of life. There were no statistically significant differences between experimental and control groups in any of the items (Fig. 2). When examining total number of items (range 0-6) by group, both were equivalent, with 76% of the control group and 75.5% of the experimental group having no aggressiveness of care items reported.
Fig. 2.
Comparisons of aggressiveness of care indices for those who died (n=214). Note: SEER data as reported by Earle et al.(5) and VAH data as reported by Gonsalves et al. (4).
Table 2 reports specific rates for selected individual items in the aggressiveness of care index. There was no statistically significant difference between groups for number of hospital admissions, nor hospital length of stay. Using multiple regression, when controlling for demographic and clinical differences that had been found to exist between the groups, we found no statistically significant change in R2 for hospital admissions (P=0.86) or for hospital days (P=0.89). Only race (β=0.31, P=0.04) and survival expectation (β= -0.01, P=0.02) made significant contributions to predicting number of hospital admissions.
Table 2.
Comparison of Outcome Variables Between Experimental and Control patients (N=610)
| Variable | Control (n=310) | Experimental (n=251) | F | P |
|---|---|---|---|---|
| Mean (SD) 95% CI | Mean (SD) 95% CI | |||
| Number of Hospitalizations | 0.99 ( 1.4) 0.84 – 1.15 | 0.97 ( 1.5) 0.79 – 1.15 | 0.03 | 0.86 |
| Days of Hospitalizationa | 8.08(13.6) 5.9– 10.3 | 8.34 (8.1) 6.84 – 9.84 | 0.03 | 0.85 |
| Days of Hospiceb | 37.8(52.2) 24.9– 50.6 | 37.6 (48.2) 26.9 – 48.3 | 0.001 | 0.98 |
| Median | 19 days | 22 days |
| n (%) | n (%) | χ 2 | P | |
|---|---|---|---|---|
| Hospice Referral:c Yes | 74 (85.1) | 91 (89.2) | 0.72 | 0.39 |
Sample size for only those who were hospitalized: control=150, experimental=115.
Sample size for only those who received hospice care: control=66, experimental=81.
Sample size for those who died: control=87, experimental=102.
Health-Related Quality of Life
Multiple regression was used to examine the relationship between group and HRQOL (using FACT-G) while controlling for race, gender and survival expectation. Group was not statistically significant for any of the four time points (enrollment, three months, nine months, and 15 months later). Overall R2 for each model was low, ranging from 0.03-0.04; gender and survival expectation made significant contributions to explaining HRQOL at all time points. Finally, given the analytic issues associated with missing data (death, attrition) and the repeated measures for HRQOL, we used a linear mixed effects modeling approach to further explore the relationship between HRQOL and group. Using this method, we evaluated the mean change in FACT-G with treatment group, while stratifying by survival expectancy. Survival expectancy was dichotomized into low survival (0-15.9) and high (16-72) using the median score (15.9) as the cutoff point for classification.
Of primary interest was the three-way interaction between treatment group, survival expectancy, and time. The three-way interaction was not significant (P=0.360). However, the survival expectancy by time interaction and survival expectancy main effect (β= 10.738) were significant (P < 0.001). The changes in FACT-G score differed over time for patients with high vs. low survival expectancies. Not surprisingly, patients with higher survival expectancy had higher FACT-G scores (better HRQOL). The survival expectancy by treatment group interaction was close to significance (P= 0.079). Subjects with a high survival expectancy in the treatment group (β = 5.132) had higher FACT-G scores, compared with the other three groupings of survival expectancy and treatment group.
Fig. 3 displays the mean of FACT-G over time by treatment group, while stratifying for survival expectancy, for a graphical summary of the trajectories of FACT-G as a function of treatment and survival expectancy. In order to examine the data without the confounding effect of those who died during the study, we ran the same analysis in the subgroup of patients who survived over the course of the study (n = 396). We included the baseline covariate for FACT-G in the model to evaluate if an individual in the treatment group with a high level of survival expectancy would be expected to change more (or less) than an individual with other levels of survival expectancy and group assignment, given that they had the same baseline measure. The three-way interaction was still not significant (P=0.470), but survival expectancy by group interaction (β= 5.102, P = 0.044) was significant. For patients who survived over the course of the study, changes in FACT-G score were greater over time for patients with high survival expectancy in the treatment group compared with all other patients.
Fig. 3.
FACT-G timeplot stratified for group and survival expectancy (n=499).
Discussion
The primary purpose of this trial was to test an approach to improving the quality of care for patients with advanced cancer, as reflected by increased referrals to hospice and reduced use of aggressive and ineffective interventions at the end of life. Our findings demonstrated no significant effect of the structural change in the system of care on quality of care indicators. Five-year survival probability demonstrated the most significant effect on aggressiveness of care outcomes. Improvement in quality of life over time was associated with the intervention among the dichotomized subgroup with the higher survival expectancy.
There are several limitations to and methodological implications of our findings. First, after the study was well underway, we learned that the performance of the study site on the quality indicators at baseline was more positive than rates and frequencies reported from other studies and from other cancer centers in the geographic region. Comparing the study site's data reported in the recent Dartmouth Atlas Report of end-of-life cancer care in the years 2003-2007 with the other six academic medical centers in Ohio, the study site showed better performance on seven of the eight indices (12). For example, the mean percent of patients enrolled in hospice at the time of death for the six other centers was 61.5%, whereas the study site reported 70.6%. The study site performance on the aggressiveness of care indices, both in the control and intervention groups, was also more positive than the performances reported by others (4-7). This high baseline performance likely reduced the potential for significant effect of the intervention.
Second, the intervention was designed as a structural change in the system of care and the actual “dose” and precise components of the intervention were non-standardized. Rather than instituting a protocol-directed, fixed, consistent package of contacts and support services, the intervention team attempted to tailor their services to the needs and preferences of each patient and family. Some patients in the intervention group received only minimal contacts because they indicated either few needs or a preference to use other resources, and thus probably received care quite similar to the patients in the control group. Although this is an important limitation and clearly diminished the likelihood of finding an overall effect, it was our intention to mimic real-world conditions, in which structured, fixed interactions with patients cannot be rigidly standardized.
The results of the final analysis indicated that there is a subgroup (those with greater survival expectancy) among patients with advanced disease that is able to benefit from integrating the interdisciplinary support services of the CST, and another group that does not appear to derive quality-of-life benefit (those with low five-year survival expectancy). This is somewhat counterintuitive in that the patients with the more lethal disease, who reported a lower HRQOL at enrollment, might be expected to show improvement with the intervention. It is possible that these patients, because of the severity of their disease, were well recognized by the regular cancer center staff as having great need, and were already receiving intensive support; thus, the addition of the CST services did not add measureable benefit.
The negative findings regarding aggressiveness of care indices mirror, to some extent, those of other researchers. Temel et al. randomized 151 patients with metastatic non-small cell lung cancer to receive early palliative care or standard oncologic care (8). They reported an increase in survival duration and a greater increase in quality of life at 12 weeks but no differences in rates of hospitalization, emergency room visits, or duration of hospice. Bakitas et al. provided four education and problem-solving sessions, followed by monthly telephone follow-up to patients with a variety of advanced cancers and reported a significantly higher quality of life for the intervention group, but no differences in days of hospitalization, ICU admissions, or emergency room visits (9).
Conclusion
Our investigation contributes to the evidence base for how to assure expert care for patients facing end-of-life transitions. First, this study adds to the evidence that dedicated support or palliative services, in combination with expert oncologic care, can improve the quality of life of some patients with advanced cancer. The surprising finding that, among the total sample of patients with advanced cancers, it was the subgroup with the relatively better prognosis and better baseline HRQOL scores who demonstrated the most benefit from the intervention, may suggest that targeting only the patients with the most apparent distress may be missing opportunities for improvement in the care of many others.
The similarity of our findings to others regarding lack of effect on aggressiveness of care indices is a cautionary note to others seeking to demonstrate the cost-effectiveness of instituting palliative care programs in an outpatient cancer center. Although there have been multiple reports of the success of palliative care programs in reducing aggressiveness of care, and thus costs of care, in the inpatient setting, there is less evidence that similar gains can be realized in outpatient settings (13). Evaluating cost-effectiveness is likely to be a required and appropriate aspect of program development, but our experience points to the value of comparisons with national benchmarking data as well as the critical importance of in-depth inclusion of non-economic outcomes, particularly quality-of-life measures.
Interpreting our findings and those of others regarding aggressiveness of care measures should be done with caution. Some of the quality standards, such as hospitalization in the last 30 days, are not always indicators of inappropriately aggressive care. Depending on the patient's home situation, available resources, or reason for hospitalization, a hospital admission may represent the optimal intervention. Similarly, although access to hospice only days before death does raise concern that there will be limited opportunity to benefit from the expertise of hospice care, some patients will not want nor accept hospice referral until quite late in the illness, and some will never elect hospice. Thus, comparisons with national benchmark data still require consideration of differences in patient characteristics, such as socioeconomic status, race, cultural values, and availability of resources in the specific community.
Finally, the methodological and analytic insights we gained in this study have implications for future research on the effectiveness of palliative and supportive care programs. Although palliative care interventions to improve quality of life during active treatment are increasingly available, there is evidence that they are not routinely integrated in cancer care. Hui et al. reported, from a survey of National Cancer Institute- and non-National Cancer Institute-designated cancer centers, that the mean duration between referral to palliative care and death for inpatient consultation teams was seven days, and for outpatient clinics was 90 days (14). This suggests that palliative care referrals are not fully nor routinely integrated in cancer care and referrals are triggered only in the late phase of illness. Expansion of such programs should continue to be a target for improvement in care delivery systems. Our study demonstrates the complexity of measuring and evaluating the effects of an integrated program and the importance of using sophisticated analytic approaches that account for relevant baseline conditions in order to provide the evidence needed to sustain investment in these essential services.
Acknowledgments
Funding for this project was obtained from the National Institute of Nursing Research and the National Cancer Institute (NR018717). The content is solely the responsibility of the authors and does not necessarily represent the views of the National Institutes of Health. The trial was registered on ClinicalTrials.gov (NCT 00684801).
The authors would like to acknowledge the dedication and skill of the members of the Cancer Support Team, whose outstanding work is described here (Helen Foley, MSN, RN, Regan Demshar, MSN, RN, Julia Schnurrenberger, MSN, RN, Judith Wolen, MSW, Lauren Garvey, MSW, and Reverend Sally Wile), as well as the support of the staff of the Seidman Cancer Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have any conflicts of interests to disclose.
References
- 1.American Cancer Society Cancer . Facts & figures 2012. American Cancer Society; Atlanta, GA: 2012. [Google Scholar]
- 2.Committee on Care at End of Life. Institute of Medicine . In: Approaching death: Improving care at the end of life. Field MJ, Cassell CK, editors. National Academies Press; Washington, DC: 1997. [PubMed] [Google Scholar]
- 3.National Cancer Policy Board. Institute of Medicine. National Research Council . In: Ensuring quality cancer care. Hewitt M, Simone JV, editors. National Academies Press; Washington, DC: 1999. [PubMed] [Google Scholar]
- 4.Gonsalves WI, Tashi T, Krishnamurthy J, et al. Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran's Affairs cancer population. Palliat Med. 2011;14:1231–1235. doi: 10.1089/jpm.2011.0131. [DOI] [PubMed] [Google Scholar]
- 5.Earle CC, Landrum MB, Souza JM, et al. Aggressiveness of cancer care near the end of life: is it a quality-of-care issue? J Clin Oncol. 2008;26:3860–3866. doi: 10.1200/JCO.2007.15.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Earle CC, Neville BA, Landrum MB, et al. Trends in the aggressiveness of cancer care near the end of life. J Clin Oncol. 2004;22:315–321. doi: 10.1200/JCO.2004.08.136. [DOI] [PubMed] [Google Scholar]
- 7.Earle CC, Neville BA, Landrum MB, et al. Evaluating claims-based indicators of the intensity of end-of-life cancer care. Int J Qual Health Care. 2005;17:505–509. doi: 10.1093/intqhc/mzi061. [DOI] [PubMed] [Google Scholar]
- 8.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 9.Bakitas M, Lyons KD, Hegel MT, et al. Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer. JAMA. 2009;302:741–749. doi: 10.1001/jama.2009.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Victorson D, Barocas J, Song J, Cella D. Reliability across studies from the functional assessment of cancer therapy-general (FACT-G) and its subscales: a reliability generalization. Qual Life Res. 2008;17:1137–1146. doi: 10.1007/s11136-008-9398-2. [DOI] [PubMed] [Google Scholar]
- 11.SAS Institute Inc. SAS/STAT® 9.22 user's guide. SAS Institute Inc.; Cary, NC: 2010. [Google Scholar]
- 12.Goodman DC, Fisher ES, Chang C, et al. Quality of end-of-life cancer care for Medicare beneficiaries: a report of the Dartmouth Atlas Project. Dartmouth College; Dartmouth, NH: 2008. [Google Scholar]
- 13.Morrison RS, Penrod JD, Cassel JB, et al. Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med. 2008;168:1783–1790. doi: 10.1001/archinte.168.16.1783. [DOI] [PubMed] [Google Scholar]
- 14.Hui D, Elsayem A, De La Cruz M, et al. Availability and integration of palliative care in US cancer centers. JAMA. 2010;303:1054–1061. doi: 10.1001/jama.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]