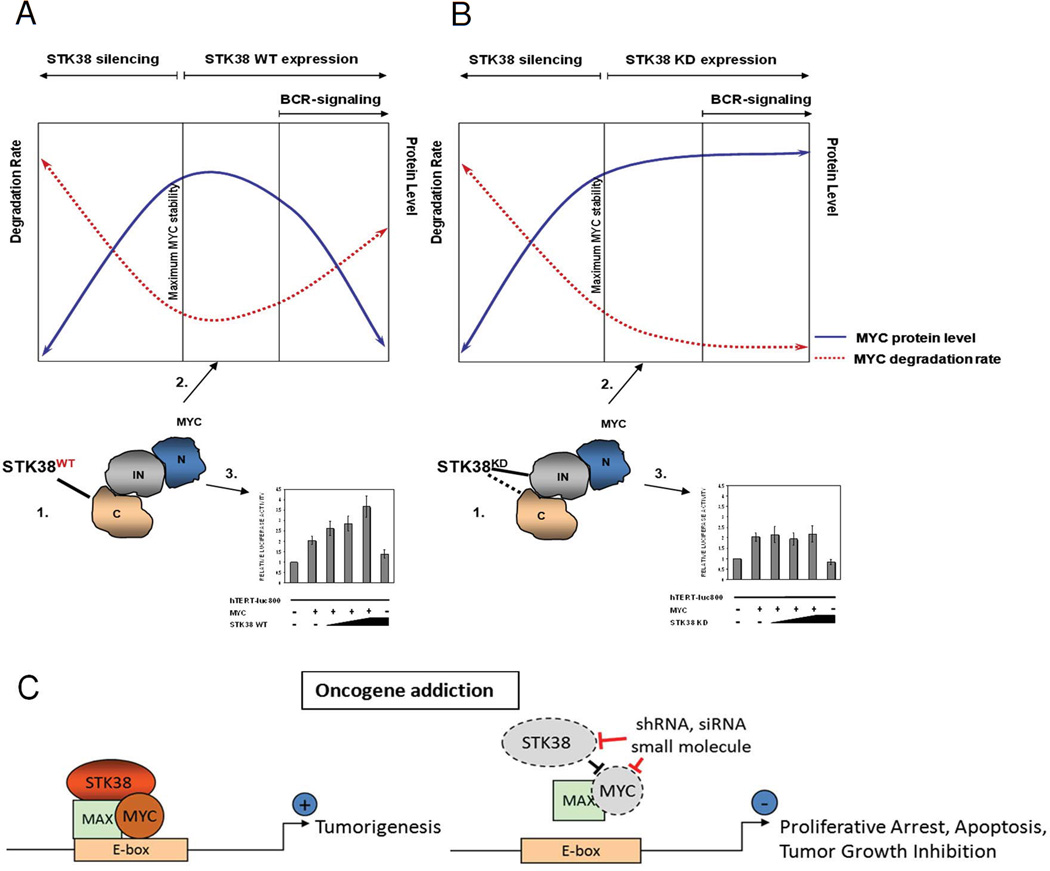
Figure 6. Model of STK38 kinase-activity-dependent modes of MYC modulation.
STK38 kinase affects MYC at several different cellular levels: 1. protein-protein interaction, 2. protein turnover and 3. transcriptional activity. (A) Kinase active STK38 binds to the C-terminus of MYC protein. Overexpression of the kinase increases MYC protein degradation rate and reduces MYC stability. Increased MYC turnover is associated with increased MYC transcriptional activity. Induction of BCR-signaling by anti-IgM crosslinking activates STK38 which causes rapid MYC degradation and induces cell apoptosis; (B) STK38-KD form interacts with MYC through an internal domain of MYC (145–353 aa) and partially C-terminus. Overexpression of STK38-KD reduces MYC protein degradation rate and stabilizes MYC protein. Reduction in MYC protein turnover is associated with lack of effect on transcriptional activity of MYC. BCRsignaling induced by anti-IgM crosslinking is disrupted by mutated inactive STK38 and therefore it cannot affect MYC turnover or transcriptional activity and cells are spared from apoptosis; silencing of endogenous STK38 results in increased MYC degradation rate followed by cell death. (C) Suppression of STK38 elicits oncogene addiction in MYC-driven tumors. STK38 stabilizes MYC protein and regulates MYC transcriptional activity in MYC-addicted tumors (left panel). Pharmacological targeting of STK38 by shRNA, siRNA or small molecule inhibitors, rapidly reduces MYC stability, leading to cell growth arrest and apoptosis (right panel).