Abstract
The dentate gyrus of the hippocampus continues to produce new neurons throughout adulthood. Adult neurogenesis has been linked to hippocampal function, including learning and memory, anxiety regulation and feedback of the stress response. It is thus not surprising that stress, which affects hippocampal function, also alters the production and survival of new neurons. Glucocorticoids, along with other neurochemicals, have been implicated in stress-induced impairment of adult neurogenesis. Paradoxically, increases in corticosterone levels are sometimes associated with enhanced adult neurogenesis in the dentate gyrus. In these circumstances, the factors that buffer against the suppressive influence of elevated glucocorticoids remain unknown – their discovery may provide clues to reversing pathological processes arising from chronic exposure to aversive stress.
Introduction
The granule cell population of the dentate gyrus is produced in three distinct phases occurring during gestation, the early postnatal period and in adulthood. During the embryonic period, new neurons arise from the ventricular zone and migrate across the hippocampal rudiment to populate the incipient dentate gyrus (Schlessinger et al., 1975; Altman & Bayer, 1990). Progenitor cells also migrate into this region and continue to produce new neurons well into the postnatal period – these new granule cells help to form the granule cell layer (Schlessinger et al., 1975; Altman & Bayer, 1990). In young adulthood, progenitor cells are located on the border of the granule cell layer and hilus, a region called the subgranular zone (sgz). These cells divide and produce new granule cells throughout adult life. Although the rate of adult neurogenesis slows considerably with advancing age (Seki & Arai, 1995; Kuhn et al., 1996; Simon et al., 2005; Leuner et al., 2007), some new granule cell production is evident even in the dentate gyrus of the very old.
Adult neurogenesis appears to be a general phenomenon of mammals, being reported in a wide range of species. Although the majority of data on adult neurogenesis come from studies using rats and mice (Cameron & McKay, 1999; Snyder et al., 2009b), new granule cell production has been shown to occur in the dentate gyrus of dogs (Hwang et al., 2007; Cotman and Head, 2008), foxes (Amrein & Slomianka, 2010), tree shrews (Gould et al., 1997; Simon et al., 2005), marmosets (new world monkeys) (Gould et al., 1998; Leuner et al., 2007), macaques (old world monkeys) (Gould et al., 1999a; Perera et al., 2007; Kordower et al., 2010) and humans (Eriksson et al., 1998; Knoth et al., 2010). In fact, the only mammals investigated in which adult neurogenesis is either absent or occurs at a very low rate in the dentate gyrus are certain types of bats (Amrein et al., 2007). Taken together, these findings suggest that with rare exceptions, adult neurogenesis is a common feature of the mammalian dentate gyrus. The wide range of species in which adult neurogenesis occurs and the relatively large number of new neurons produced at least in the species for which adequate quantitative data exist (rats, mice, marmosets) suggest that this form of structural plasticity may play an important role in hippocampal function. For this reason, the regulation and function of adult neurogenesis has received focused attention by the Neuroscience community over the past decade.
Numerous studies have attempted to characterize the production of new neurons in the dentate gyrus of adults. Adult neurogenesis can be divided into three main cellular events: cell proliferation, neuronal differentiation, and cell survival (Fig 1) (Christie & Cameron, 2006). Each of these events has been well-characterized, at least in studies of rodents, and each provides a plastic process that has the potential to be influenced by stress and glucocorticoids.
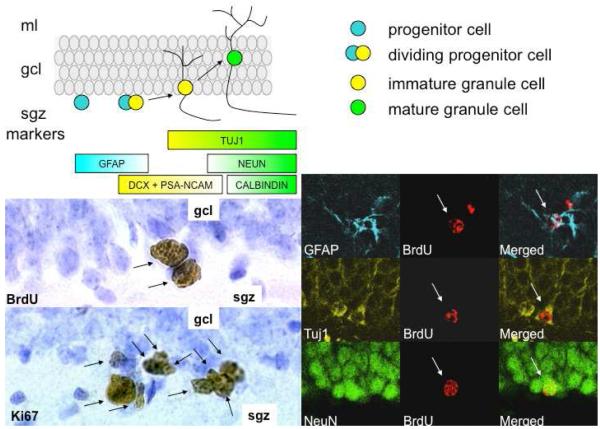
Figure 1.
Top left: schematic diagram of adult neurogenesis in the dentate gyrus of the hippocampus. Progenitor cells in the subgranular zone (sgz) divide and produce daughter cells. Most of these cells differentiate into excitatory granule cells and integrate into the granule cell layer as they differentiate. At different timepoints during their maturation, new cells express specific biochemical markers, listed below the schematic diagram. Photomicrographs (bottom left) show BrdU labeled cells (arrows, top), and Ki67 labeled cells (arrows, bottom). BrdU is an exogenously applied marker that labels cells in S phase while Ki67 is an endogenous marker of actively cycling. Confocal microscopic images (bottom right) show cells labeled with BrdU (red) and a marker of astroglia (GFAP), immature and mature neurons (Tuj1) and mature neurons (NeuN).
Cell proliferation refers to the division of progenitor cells located in the sgz of the dentate gyrus. Granule cell progenitors have the morphological characteristics of radial glia (Seri et al., 2001) and express glial fibrillary acidic protein (GFAP), an astroglial marker. Progenitor cells continue to express GFAP at the time of cell proliferation. Neuronal differentiation refers to the selection and emergence of a neuronal fate by some of the daughter cells. In the dentate gyrus, the majority of new cells differentiate into neurons (in the rodent, the percentage varies in the literature between 80-95%, depending on factors such as species, animal age, location of granule cells, and stage of development of cells, Cameron et al., 1993b; Cameron & McKay, 2001; Brown et al., 2003; Snyder et al., 2009). A smaller percentage (~10), differentiate into glia (Cameron et al., 1993b; Steiner et al., 2004). New glial cells continue to express GFAP while undergoing structural differentiation into mature astrocytes. New neurons stop expressing GFAP and instead express markers for immature neurons, such as doublecortin (DCX), polysialated neuronal cell adhesion molecule (PSA-NCAM) and class III beta-tubulin (Tuj1). While new neurons continue to express TuJ1 as they mature, these cells ultimately stop producing DCX and PSA-NCAM and begin to make proteins specific to mature granule cells, like Neuron specific enolase (NSE), Neuronal nuclei (NeuN) and Calbindin (Fig. 1). It should be noted that the time course of biochemical maturation of new neurons in the dentate gyrus varies among species, even within rodents. For example, new neurons in the adult rat appear to differentiate more rapidly than those in the adult mouse (Snyder et al., 2009a). In addition to biochemical changes that accompany neuronal differentiation, new neurons undergo structural and electrophysiological changes as they transition from immature to mature. Within a few weeks of mitosis, new neurons develop morphological features of granule cells. New granule cells grow characteristic dendritic trees extending toward the molecular layer (Ribak et al., 2004), elaborate axons toward the CA3 region of the hippocampus (Hastings & Gould, 1999; Zhao et al., 2006), and generate action potentials (van Praag et al., 2002). Initially, new neurons respond to GABA, the main inhibitory neurotransmitter of mature granule cells, with excitation (Ge et al., 2006). As new neurons mature, Cl- channels on the granule cell membrane mature and GABA has an inhibitory effect. New neurons also show enhanced synaptic plasticity during maturation (Snyder et al., 2001; Ge et al., 2007) compared to mature granule cells. This lack of inhibition and increased plasticity make new neurons an ideal substrate for influencing hippocampal function.
Cell survival refers to the maintenance of new neurons and their permanent incorporation into the hippocampal circuitry. Some new granule cells survive for very long periods of time (Dayer et al., 2003) but that is not the case for all such cells produced in adulthood. In control rodents, a relatively large percentage of new neurons do not survive past a few weeks in the rodent (Dayer et al.,2003). The survival of new neurons can be influenced by environmental factors, including stress, suggesting that data on cell survival may be confounded by the relatively deprived conditions of standard laboratory life. Studies have shown that all three stages of adult neurogenesis, cell proliferation, neuronal differentiation and cell survival, can be influenced by stress, learning and environmental enrichment (Leuner & Gould, 2010) but the majority of evidence points to stress effects on cell proliferation.
Effects of Stress on Adult Neurogenesis
Acute Stressors
Several studies have investigated the effects of stress on adult neurogenesis – these reports have varied in the stressor used and the duration of its application (Table 1). For acute stress, a single episode of stressful experience, some conflicting data exist but the overall result appears to be that stress inhibits adult neurogenesis by lowering the rate of cell proliferation. Subordination stress in adult tree shrews and marmosets results in a decrease in cell proliferation in the dentate gyrus (Gould et al., 1997; 1998). Similar results have been demonstrated for adult mice exposed to social defeat (Yap et al., 2006; Lagace et al., 2010). However, one study reported that acute exposure to a dominant conspecific does not affect cell proliferation in rats, but instead decreases survival of new neurons (Thomas et al., 2007). A likely reason for the lack of effect on cell proliferation in this study was that new cells were labeled with BrdU before the stressful experience so it was not a direct test of stress effects on proliferating cells. Indeed, a large number of studies examining other types of stressors suggest that acute stress can have a suppressive effect on cell proliferation in the dentate gyrus.
Table 1.
Summary of the effects of different experiences that elevate glucocorticoids on cell proliferation, neuronal differentiation, and cell survival in the dentate gyrus. In general, stressful aversive experiences like social defeat, physical restraint and fox odor exposure, have a suppressive effect on adult neurogenesis, while rewarding experiences like running, environmental enrichment, and sexual experience exert a stimulatory effect on adult neurogenesis.
| Experience | Cell Proliferation | Neuron Differentiation | Neuron Survival |
|---|---|---|---|
| Acute Social Defeat | Decrease (tree shrew; mamoset) | NA | Decrease (rat) |
| Chronic Social Defeat | Decrease (tree shrew; rat; mouse) | Decrease (mouse) | Decrease (tree shrew; rat) |
| Acute Predator Odor | Decrease (rat: male) No change (rat: female) |
NA | NA |
| Acute Physical Restraint | Decrease (rat) No Change (rat) Increase (mouse) |
NA | NA |
| Chronic Physical Restraint | Decrease (rat) No Change (rat) |
NA | Decrease (rat) Increase (mouse) |
| Acute Electric Shock | Decrease (rat) | NA | NA |
| Chronic Electric Shock | Decrease (rat) | Decrease (rat) | Decrease (rat: male) Increase (rat: female) |
| Difficult Learning Paradigm | Decrease (rat) No Change (rat) |
NA | Decrease (rat) |
| Chronic Multiple Mild Stress | Decrease (rat) | Decrease (rat) | Decrease (rat) |
| Running | Increase (rat; mouse) | Increase (rat; mouse) | Increase (rat; mouse) |
| Environmental Enrichment | Increase (rat; mouse) | Increase (rat; mouse) | Increase (rat; mouse) |
| Sexual Experience | Increase (rat) | NA | Increase (rat) |
Exposure to the odors of natural predators activates the HPA axis and produces anxiety-like behavior in rats. Acute stress through exposure to trimethylthiazoline (TMT), a component in fox feces, decreases cell proliferation and differentiation of immature neurons in the dentate gyrus (Hill et al., 2006; Kambo & Galea, 2006; Mirescu et al., 2004; Tanapat et al., 2001). This decrease is not due solely to novel odor experience, as other novel odors do not reduce cell proliferation (Tanapat et al., 2001). One study found no change in cell proliferation following predator odor exposure (Thomas et al., 2006), however, as with the social stress study described previously (Thomas et al., 2007), new cells were labeled during the stressful experience, instead of following it, and therefore is not an accurate test of the effects of stress on cell proliferation.
The effects of physical restraint are somewhat complicated to interpret and not as straight-forward as the stress effects of social dominance and predator odor effects on adult neurogenesis. Several studies have suggested that acute restraint lasting two to six hours has no effect on cell proliferation in adult rats (Kee et al., 2002; Pham et al., 2003; Rosenbrock et al., 2005). However, one study showed that three hours of restraint decreases cell proliferation in the adult rat (Bain et al., 2004). The same study showed that an increase in cell proliferation was seen in adult mice after acute restraint. It has been suggested that comparisons among different studies using nonstandardized methodologies of physical restraint are impossible because of the variations in intensity, duration, and frequency of restraint across rodent species and strains (Buynitsky & Mostofsky, 2009).
Electric shock to the foot or tail activates the HPA axis and induces anxiety-like behavior in rodents. Acute exposure to electric shock decreases cell proliferation in the dentate gyrus of adult rats (Malberg & Duman, 2003). One study showed a delayed decrease in cell proliferation seven days following shock trials, after the increases in glucocorticoid levels had gone back down to baseline (Fornal et al., 2007), suggesting a more complicated relationship may exist between electric shock and cell proliferation.
Chronic Stressors
Chronic stress paradigms typically utilize daily stressors over the course of a few days to several weeks. Chronic social stress decreases cell proliferation in tree shrews (Czeh et al., 2001; Czeh et al., 2002; Simon et al., 2005), rats (Czeh et al., 2007), and mice (Ferragud et al., 2010), where subordinate behavior is negatively correlated with cell proliferation rates (Mitra et al., 2006). Chronic social stress also decreases differentiation of new neurons in mice (Ferragud et al., 2010), although this may reflect changes in proliferation, and survival of new neurons in tree shrews (Czeh et al., 2002) and rats (Czeh et al., 2007). Chronic restraint stress has been shown to decrease or not change cell proliferation in adult rats (Pham et al., 2003; Rosenbrock et al., 2005). Chronic restraint stress has also been shown to reduce survival of new neurons in rats (Pham et al, 2003) but enhance survival of new neurons in mice (Snyder et al., 2009b). Chronic electric shock decreases both cell proliferation and neuronal differentiation (although this latter effect may stem from reduced cell proliferation as well) in adult rats (Dagyte et al., 2009).
Although various studies have suggested that learning increases neurogenesis in the adult dentate gyrus (Gould et al., 1999b; Leuner et al., 2004; 2006; Epp et al., 2010), when learning is difficult or stressful, it can have a negative impact on cell proliferation (Aztiria et al., 2007). Stress related to using novel testing paradigms decreases cell proliferation even though learning occurs (Ehninger & Kempermann, 2006). Step-wise increases in task difficulty do not change cell proliferation, but decrease survival of new neurons in the adult rat dentate gyrus (Epp et al., 2010).
Chronic use of multiple mild stressors is a model of animal depression, as animals tend to develop symptoms of learned helplessness over the course of days and weeks. Mild stressors commonly used include cold-water swim, immobilization, social isolation, food and water deprivation, chronic illumination, white noise exposure, tail pinch, tilted or shaken cage, and electric shock, although experiments typically do not use all of the above. Multiple mild stressors decrease cell proliferation directly following a stressor, although this effect can be short-lived (Xu et al., 2007). Neurons born prior to mild stressor exposure show diminished differentiation and survival (Lee et al., 2006; Oomen et al., 2007). Overall, the results suggest that chronic stressful experience decreases adult neurogenesis by influencing cell proliferation, neuronal differentiation and cell survival although the effects vary depending on the study perhaps because of differences in stressor, species or strain.
Age, Species, and Sex Differences
Adult neurogenesis declines steadily with age in the hippocampus of every species in which this has been examined, including rats, mice, tree shrews, dogs and marmosets (Seki & Arai, 1995; Kuhn et al., 1996; Cameron & McKay, 1999; Simon et al., 2005; Leuner et al., 2007). Some evidence suggests that stress effects on adult neurogenesis may be greater in the aged animal than the young animal. There is a greater decrease in cell proliferation of aged tree shrews compared to young adult tree shrews following social stress (Simon et al., 2005).
Baseline differences in adult neurogenesis in the dentate gyrus exist among species. Most notably, there is greater production of new neurons in rats compared to mice; these neurons differentiate faster and are more functionally significant in the hippocampus of rats than mice (Snyder et al., 2009a). As mentioned above, acute restraint stress decreases cell proliferation in rats but increases cell proliferation in mice (Bain et al., 2004). Species differences in adult neurogenesis may alter the effects of stress on adult neurogenesis.
Robust sex differences in baseline adult neurogenesis in the dentate gyrus have not been reported, although estrous cycle differences exist in female rats (Tanapat et al., 1999), but not in female mice (Lagace et al., 2007). However, females and males may differ in how adult neurogenesis is affected by stressful experiences. The reduction in cell proliferation in adult male rats after fox odor exposure is not seen with female rats (Falconer & Galea, 2003). Male rats show decreases in the survival of new neurons following chronic electric shock, but female rats show increases in the survival of new neurons following chronic electric shock (Westenbroek et al., 2004). These results only appear during periods of social isolation. When rats are group-housed, the differences disappear between males and females. Prenatal stress can affect baseline neurogenesis rates when pups mature to adulthood. Male rats that were stressed prenatally have suppressed baseline survival of new neurons, while there is no change in female rats that were stressed prenatally (Zuena et al., 2008). Early weaning results in greater suppression of cell proliferation and survival of new neurons in adult male versus adult female mice (Kikusui et al., 2009). These results suggest that differences across age, species and sex exist in various effects of stressful experiences on adult neurogenesis.
Mechanisms underlying stress effects on adult neurogenesis
Adrenal steroids
Stress is accompanied by HPA activity, which results in the release of glucocorticoids into the blood. In general, glucocorticoids appear to inhibit adult neurogenesis in the dentate gyrus. Exogenous administration of corticosterone to rodents produces a decrease in the number of proliferating cells and surviving new granule neurons (Cameron & Gould, 1994; Wong & Herbert, 2006; Brummelte & Galea, 2010a). The suppressive action of corticosterone on cell proliferation seems to occur independent of sex (Brummelte & Galea, 2010a) and reproductive status (Brummelte & Galea, 2010b). By contrast to the negative actions of glucocorticoids on adult neurogenesis, removal of the adrenal glands by adrenalectomy (ADX), stimulates cell proliferation and adult neurogenesis in the dentate gyrus (Gould et al., 1992; Cameron & Gould, 1994). Taken together, these findings suggest that the rate of cell proliferation and adult neurogenesis in the dentate gyrus of adult rodents can be regulated by the levels of circulating glucocorticoids. Since glucocorticoid injections produce similar effects on adult neurogenesis as stress, it is likely that the stress-induced increases in glucocorticoid levels are responsible for the stress-induced decreases in adult neurogenesis. Indeed, inhibitory effects of fox odor exposure on cell proliferation can be blocked by preventing the stress-induced rise in glucocorticoids (Tanapat et al., 2001). It remains unknown, however, whether these effects are mediated directly via actions of adrenal steroids on progenitor cells or whether they occur indirectly through some unknown factor.
Adrenal steroid effects: direct or indirect?
Glucocorticoids bind to two main types of receptors in the brain, the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (Reul & de Kloet, 1985). Granule cells in the dentate gyrus express both subtypes of adrenal steroid receptors. Because MRs have higher affinity for glucocorticoids than GRs, MRs are more sensitive to circadian changes in glucocorticoids while GRs respond more to stress-induced elevations in glucocorticoids (de Kloet et al., 1998).
Although most new neurons express both GR and MR after 4 weeks of maturation, relatively few progenitor cells express glucorticoid receptors (Cameron et al., 1993a; Garcia et al., 2004a). This raises the possibility that adrenal steroid-mediated changes in the rate of cell proliferation in the dentate gyrus occur indirectly. There are several possible mechanisms whereby such an indirect effect could occur. For instance, glucocorticoids might affect neurogenesis by influencing neighboring, more mature, granule neurons. This could occur either by altering the survival of granule cells directly or by affecting their afferent inputs.
With regard to the first possibility, ADX results in massive death of mature granule cells in the dentate gyrus (Sloviter et al., 1989; Gould et al., 1990). Replacement of ADX rats with aldosterone, a mineralocorticoid that binds with high affinity to MRs, is sufficient to protect the dentate gyrus from cell death (Woolley et al., 1991), suggesting that regular activation of MRs is important for normal dentate gyrus function. These findings suggest that dying mature granule cells may provide signals that stimulate the proliferation of progenitor cells. In this regard, it is relevant to note that direct destruction of the dentate gyrus, via chemical or mechanical lesion, leads to an increase in the production of new neurons (Gould & Tanapat, 1997). The link between cell survival and cell proliferation has not been extensively explored in the dentate gyrus but several reports suggest that neuronal death can stimulate adult neurogenesis in many other brain regions, including the neocortex and striatum (Gould, 2007). It is possible that dying cells produce a chemical signal that is mitogenic. Alternatively, mature intact neurons may provide an anti-mitotic signal that is lost when cells die leading to an increase in cell proliferation. The specific signals, however, remain unknown.
An additional, but not mutually exclusive, possibility is that neurogenesis is affected indirectly through adrenal steroid actions on granule cell afferents. Lesion of the entorhinal cortex, one of the main afferent populations to the dentate gyrus, stimulates the production of new neurons (Cameron et al., 1995). Likewise, blockade of NMDA receptors, glutamate receptors involved in perforant path-granule cell synapses, increases adult neurogenesis (Cameron et al., 1995; Maekawa et al., 2009). Moreover, manipulation of cholinergic inputs, via either neurotoxin or pharmacological intervention, alters the rate of adult neurogenesis (Kotani et al., 2006; Frechette et al., 2009). Although not directly explored in the context of adrenal steroids, these afferent populations contain adrenal steroid receptors and may be one of the intermediate steps between alterations in hormone levels and changes in the production of new neurons.
Cytokines
Exposure to certain types of stressors, but not other, increases the levels of cytokines such as interleukin-1 (IL-1) in the periphery and brain (Grippo et al., 2005; Deak et al., 2005). Increased levels of IL-1 can further sensitize HPA axis responses to subsequent stressor exposures (Schmidt et al., 2003; Johnson et al., 2004). Interleukin-1 (IL-1) is a pro-inflammatory cytokine which is a member of a family of immune factors that communicate inflammation to the central nervous system. IL-1 works to stimulate glucocorticoid release by the adrenal glands (Bernton et al., 1987). This pathway raises the possibility that under certain conditions, stress may inhibit adult neurogenesis by stimulated glucocorticoid release through elevated IL-1. Inflammation, a condition associated with increased IL-1, has been shown to reduce cell proliferation and survival of new neurons in the dentate gyrus of the adult rat (Ekdahl et al., 2003). Administration of IL-1β itself decreases cell proliferation and differentiation of new neurons in the adult mouse dentate gyrus (Goshen et al., 2008; Koo & Duman, 2008). In vivo and in vitro studies suggest that progenitor cells in the SGZ have IL-1 receptors and that activation of these receptors decreases cell proliferation (Koo & Duman, 2008). Inactivation of IL-1 receptors via transgenic manipulations (Goshen et al., 2008) or pharmacological antagonists (Ben Menachem-Zidon et al., 2008) block stress-induced depressive-like behaviors and decreased cell proliferation in the dentate gyrus. These findings suggest IL-1 may underlie the stress-induced decrease in adult neurogenesis in the dentate gyrus but since available evidence suggests that not all stressors activate this pathway, the mechanism is unlikely to be universal.
Paradoxical Effects of Rewarding Experience on Adult Neurogenesis
Despite the various experiences that activate the HPA axis and produce a suppressive effect on adult neurogenesis (Table 1), there are some behaviors that activate the HPA axis but are associated with increased rates of adult neurogenesis. For example, physical exercise activates the HPA axis and increases glucocorticoid levels in the blood (Droste et al., 2003; Makatsori et al., 2003; Stranahan et al., 2006). Physical exercise also enhances cell proliferation, neuronal differentiation and survival of new neurons in the dentate gyrus of the adult mouse (Klaus et al., 2009; van Praag et al., 1999; Snyder et al., 2009b) and rat (Stranahan et al., 2006; Yi et al., 2009). Running also rescues cell proliferation in the dentate gyrus from alcohol-induced inhibition (Crews et al., 2004). This suggests that running engages mechanisms that protect progenitor cells or new neurons from the detrimental effects of elevated glucocorticoids.
Housing in an enriched environment can increase adrenal gland size (Moncek et al., 2004) and circulating glucocorticoid levels (Benaroya-Milshtein et al., 2004). However, environmental enrichment increases differentiation and survival of new neurons (van Praag et al., 1999) and buffers against aged-related decreases in neurogenesis (Kempermann et al., 2002) in adult mice. Enriched environment living also rescues stress-induced decreases in cell proliferation, neuronal differentiation and survival of new neurons in the adult rat (Veena et al., 2009a; 2009b). Again, this suggests some protective mechanism of enriched environment living that allows for neuronal growth despite elevated glucocorticoid levels.
Sexual experience also increases circulating glucocorticoid levels (Bonilla-Jaime et al., 2006). Acute sexual experience increases cell proliferation in the dentate gyrus of adult rats (Leuner et al., 2010). Chronic sexual experience increases cell proliferation and adult neurogenesis in the dentate gyrus (Leuner et al., 2010). Learning has also been shown to increase glucocorticoid levels (Leuner et al., 2004) and various studies have shown that training on certain types of learning tasks increases adult neurogenesis (reviewed in Leuner et al., 2006).
Taken in the context of the negative actions of glucocorticoids on adult neurogenesis, these findings are puzzling and raise the question of whether running, enriched environment living, sexual experience and learning have a common characteristic that permits neuronal growth despite a negative hormonal milieu. In this regard, it may be relevant that all of these experiences have a hedonic component. Running, environmental enrichment, and sexual experience are rewarding to rodents. Rats form place preferences for running wheels and mating chambers (Belke & Wagner, 2005; Tenk et al., 2009) and show increased bar pressing to gain access to wheels or receptive females (Everitt et al., 1987; Hundt & Premack, 1963). Rats show anticipatory behavior towards gaining access to an enriched environment (van der Harst et al., 2003). Other types of rewarding experiences can also promote adult neurogenesis, as intercranial self-stimulation will stimulate cell proliferation in the dentate gyrus of adult rats and mice despite elevated glucocorticoid levels (Takahashi et al., 2009). The rewarding nature of these experiences may provide some clues about mechanisms that protect the brain from the negative influences of high levels of glucocorticoids.
Rewarding social experiences are associated with the release of factors that may serve to protect against elevated glucocorticoids and actually promote neuronal growth. Among these are neuropeptides, such as endogenous opioids and oxytocin and the neuromodulator dopamine. Some evidence suggests that each of these factors is capable of stimulating the production of new neurons in the hippocampus (Morton et al., 2009; Höglinger et al., 2004; Winner et al., 2009; Lloyd et al., 2010; Koehl et al., 2008; Persson et al., 2003). It is also possible that neurotrophic factors play a role in buffering the brain from the suppressive actions of elevated glucocorticoids. For example, brain-derived neurotrophic factor (BDNF) is increased following running (Ying et al., 2005). BDNF is a factor in survival of new neurons (Sairanen et al., 2005), and blocking BDNF decreases differentiation of new neurons in the adult mouse dentate gyrus (Taliaz et al., 2010). BDNF is required for enriched environment-induced increases in cell proliferation in adult mice (Rossi et al., 2006). Vascular endothelial growth factor (VEGF) administration also increases cell proliferation in the dentate gyrus of the adult rat (Jin et al., 2002). VEGF is required for running-induced increases in cell proliferation and differentiation of new neurons in adult mice (Fabel et al., 2003), and for enriched environment-induced cell proliferation and differentiation and survival of new neurons in adult rats (Cao et al., 2004). Chronic stress decreases VEGF expression (Heine et al., 2005), so VEGF is another good potential factor in mitigating adult neurogenesis. Insulin-like growth factor 1 (IGF-1) administration increases cell proliferation in the dentate gyrus of the adult rat (Aberg et al., 2001), mediates positive neural changes in the brain following exercise (Carro et al., 2000), and is increased following antidepressant treatment (Khawaja et al., 2004), which is known to stimulate cell proliferation. Therefore, IGF-1 may also be a factor in the paradoxical effects of rewarding experiences on adult neurogenesis, although no direct evidence has supported this yet. No studies have yet examined the roles of BDNF, VEGF, or IGF-1 in sexual experience.
Potential Consequences of Stress-induced Changes in Adult Neurogenesis
The influence of elevated glucocorticoid levels and exposure to stressful experiences on adult neurogenesis raises the question of what is the functional impact of changing the rate of new neuron production in the adult hippocampus. Since the hippocampus is important for certain types of learning and memory (Moser et al., 1993; Ergorul & Eichenbaum, 2004), anxiety regulation (Bannerman et al., 2004) and shutting off the HPA axis (Herman et al., 1989; Jacobson & Sapolsky, 1991; Herman et al., 1995; Herman & Mueller, 2006), these present possible functions that may be affected by changes in adult neurogenesis. It should be emphasized at the outset of this discussion, that stress and glucocorticoids exert effects elsewhere in the hippocampus, including on the pyramidal cell population (Fig. 2). These effects, which include changes in the biochemistry, electrophysiology and structure of neurons in the CA fields, are likely to contribute to stress-induced changes in hippocampal function. The extent to which changes in adult neurogenesis, through the connections of the granule cell population to other neuronal populations (e.g., CA3 pyramidal neurons, hilar mossy cells), participate in stress-induced changes throughout the hippocampus remains unknown. However, stress effects other than those on adult neurogenesis should be kept in mind when attempting to assess the functional impact of changes in new neurons.
Figure 2.
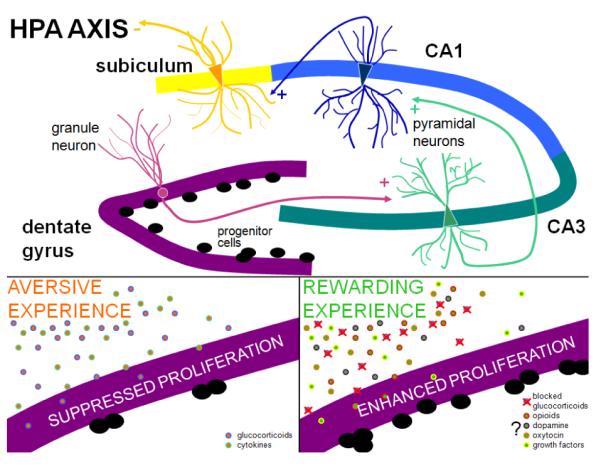
Schematic diagram of the effects of stress and glucocorticoids on principal cell types in the hippocampus. Stress causes release of glucocorticoids which bind to receptors on all principal cell types in the hippocampus. The dentate gyrus contains granule cells which have excitatory connections with pyramidal cells of the CA3 region. CA3 pyramidal cells have excitatory connections to pyramidal cells in the CA1 region, which have excitatory connections to pyramidal cells in the subiculum, the main efferent population in the hippocampus. Pyramidal cells in the subiculum ultimately exert an inhibitory influence over the HPA axis, helping the system to return to baseline after stress. Aversive experiences are known to inhibit adult neurogenesis in the dentate gyrus potentially through elevated levels of glucocorticoids and cytokines. Rewarding experiences, on the other hand, are known to stimulate adult neurogenesis in the dentate gyrus despite elevated levels of glucocorticoids. Factors that protect against elevated glucocorticoids under conditions of rewarding experience remain unknown – some potential candidates include opioids, dopamine, oxytocin and growth factors.
Most studies investigating the functional impact of new neurons in the hippocampus have tended to focus on their potential role in learning and memory. Antiproliferative agents (Shors et al., 2001; Garthe et al., 2009), irradiation (Madsen et al., 2003), and transgenic models (Garcia et al., 2004b), all ways to reduce cell proliferation in the hippocampus, have been shown to produce changes in cognitive tasks associated with the hippocampus. Decreased hippocampal neurogenesis has no effect on hippocampal-independent cued fear conditioning, but impairs hippocampal-dependent context fear conditioning (Winocur et al., 2006; Saxe et al., 2006; Warner-Schmidt et al., 2008; Imayoshi et al., 2008; Farioli-Vecchioli et al., 2008). In rats, context fear deficits do not appear until at least 4 weeks following neurogenesis ablation (Snyder et al., 2009a), suggesting that a certain degree of new neuron maturation is critical for context fear conditioning in rats. In the Morris water maze paradigm, rats show deficits in lasting retention of spatial information at least 4 weeks following ablation of hippocampal neurogenesis, but not before (Shors et al., 2002; Madsen et al., 2003; Snyder et al., 2005; Jessberger et al., 2009). In mice, the picture is less clear. Studies have shown deficits or no change in context fear conditioning and Morris water maze learning in mice of various strains, gender, and ages, from different time points following ablation (Raber et al., 2004; Rola et al., 2004; Saxe et al., 2006; Meshi et al., 2006; Imayoshi et al., 2008; Dupret et al., 2008; Zhang et al., 2008; Farioli-Vehhcioli et al., 2008; Deng et al, 2009; Garthe et al., 2009; Ko et al., 2009, Kitamura et al., 2009; Snyder et al., 2009a; Goodman et al., 2010), suggesting that strain, gender, and age-related differences in mice may exist in the time course for new neuron maturation and integration or reactions to different ablation techniques.
Recently, it has been argued that classical hippocampal-dependent learning paradigms do not accurately reflect the role of the dentate gyrus (Deng et al., 2010). Computational work suggests that the dentate gyrus may be involved specifically in pattern separation, the process where highly similar, overlapping cortical representations are separated to keep them independent in episodic memory (O’Reilly & McClelland, 1994). Some evidence suggests that new neurons may be important for the ability of the dentate gyrus to separate patterns (Deng et al., 2010). Increased adult neurogenesis has been linked to enhanced spatial pattern separation in mice (Creer et al., 2010). Conversely, ablation of new neurons in the dentate gyrus produces deficits in spatial pattern separation in mice (Clelland et al., 2009). Taken together, the available evidence suggests that stress-reduced adult neurogenesis in the dentate gyrus may have profound effects on hippocampal-dependent memory formation and learning although the specific functions affected remain undetermined.
More recent studies have linked adult neurogenesis with anxiety regulation and feedback of the stress response. Experimental manipulations associated with reduced number of new neurons in the dentate gyrus are associated with increased anxiety-like behavior (Bergami et al., 2009; Revest et al., 2009). Likewise, reduced adult neurogenesis is associated with impaired modulation of the HPA axis – corticosterone levels show a delayed return to baseline after stress in mice lacking new neurons. Furthermore, reduced neurogenesis is associated with impaired responsiveness of the HPA axis to a dexamethasone suppression test (Snyder et al., 2010). Taken together, these findings suggest that new neurons may play an important role not only in the cognitive functions of the hippocampus, but also in its anxiety and stress regulatory functions. The extent to which stress-induced reductions in adult neurogenesis contribute to increased pathological processes associated with chronic stress, such as anxiety and HPA axis dysregulation, remains to be determined.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg MA, Aberg ND, Hedbacker H, Oscarsson J, Eriksson PS. Peripheral infusion of IGF-1 selectively induces neurogenesis in the adult rat hippocampus. J Neurosci. 2000;20:2896–2903. doi: 10.1523/JNEUROSCI.20-08-02896.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Migration and distribution of two populations of hippocampal granule cell precursors during the perinatal and postnatal periods. J Comp Neurol. 1990;301:365–381. doi: 10.1002/cne.903010304. [DOI] [PubMed] [Google Scholar]
- Amrein I, Dechmann DK, Winter Y, Lipp HP. Absent or low rate of adult neurogenesis in the hippocampus of bats (Chiroptera) PLoS One. 2007;2:e455. doi: 10.1371/journal.pone.0000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I, Slomianka L. A morphologically distinct granule cell type in the dentate gyrus of the red fox correlates with adult hippocampal neurogenesis. Brain Res. 2010;1328:12–24. doi: 10.1016/j.brainres.2010.02.075. [DOI] [PubMed] [Google Scholar]
- Aztiria E, Capodieci G, Arancio L, Leanza G. Extensive training in a maze task reduces neurogenesis in the adult rat dentate gyrus probably as a result of stress. Neurosci Lett. 2007;416:133–137. doi: 10.1016/j.neulet.2007.01.069. [DOI] [PubMed] [Google Scholar]
- Bain MJ, Dwyer SM, Rusak B. Restraint stress affects hippocampal cell proliferation differently in rats and mice. Neurosci Lett. 2004;368:7–10. doi: 10.1016/j.neulet.2004.04.096. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus—memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Belke TW, Wagner JP. The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav Processes. 2005;68:165–172. doi: 10.1016/j.beproc.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O, Goshen I, Kreisel T, Menahem Y. Ben, Reinhartz E, Hur T. Ben, Yirmiya R. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Yaniv I, Kukulansky T, Raz N, Haberman Y, Halpert H, Pick CG, Hollander N. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. Eur J Neurosci. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bergami M, Berninger B, Canossa M. Conditional deletion of TrkB alters adult hippocampal neurogenesis and anxiety-related behavior. Commun Integr Biol. 2009;2:14–16. doi: 10.4161/cib.2.1.7349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernton EW, Beach JE, Holaday JW, Smallridge RC, Fein HG. Release of multiple hormones by a direct action of interleukin-1 on pituitary cells. Science. 1987;238:519–521. doi: 10.1126/science.2821620. [DOI] [PubMed] [Google Scholar]
- Bonilla-Jaime H, Vázquez-Palacios G, Arteaga-Silva M, Retana-Márquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Després S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010a;168:680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav. 2010b;58:769–779. doi: 10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Buynitsky T, Mostofsky DI. Restraint stress in biobehavioral research: Recent developments. Neurosci Biobehav Rev. 2009;33:1089–1098. doi: 10.1016/j.neubiorev.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McEwen BS, Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J Neurosci. 1995;15:4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Restoring production of hippocampal neurons in old age. Nat Neurosci. 1999;2:894–897. doi: 10.1038/13197. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993a;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, McEwen BS, Gould E. Differentiation of newly born neurons and glia in the dentate gyrus of the adult rat. Neuroscience. 1993b;56:337–344. doi: 10.1016/0306-4522(93)90335-d. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–17. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cao L, Jiao X, Zuzga DS, Liu Y, Fong DM, Young D, During MJ. VEGF links hippocampal activity with neurogenesis, learning, and memory. Nat Genet. 2004;36:827–835. doi: 10.1038/ng1395. [DOI] [PubMed] [Google Scholar]
- Carro E, Nunez A, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates effects of exercise on the brain. J Neurosci. 2000;20:2926–2933. doi: 10.1523/JNEUROSCI.20-08-02926.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Head E. The canine (dog) model of human aging and disease: dietary, environmental and immunotherapy approaches. J Alzheimers Dis. 2008;15:685–707. doi: 10.3233/jad-2008-15413. [DOI] [PubMed] [Google Scholar]
- Creer DJ, Romberg C, Saksida LM, van Praag H, Bussey TJ. Running enhances spatial pattern separation in mice. Proc Natl Acad Sci USA. 2010;107:2367–2372. doi: 10.1073/pnas.0911725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Czeh B, Michaelis T, Watanabe T, Frahm J, de Biurrun G, van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci USA. 2001;98:12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeh B, Muller-Keuker JI, Rygula R, Abumaria N, Hiemke C, Domenici E, Fuchs E. Chronic social stress inhbits cell proliferation in the adult medial prefrontal cortex: hemispheric asymmetry and reversal by fluoxetine treatment. Neuropsychopharmacology. 2007;32:1490–1503. doi: 10.1038/sj.npp.1301275. [DOI] [PubMed] [Google Scholar]
- Czeh B, Welt T, Fischer AK, Erhardt A, Schmitt W, Müeller MB, Toschi N, Fuchs E, Keck ME. Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol Psychiatry. 2002;52:1057–1065. doi: 10.1016/s0006-3223(02)01457-9. [DOI] [PubMed] [Google Scholar]
- Dagyte G, Van der Zee EA, Postema F, Luiten PG, Boer J.A. Den, Trentani A, Meerlo P. Chronic but not acute foot-shock stress leads to temporary suppression of cell proliferation in rat hippocampus. Neuroscience. 2009;162:904–913. doi: 10.1016/j.neuroscience.2009.05.053. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Ford AA, Cleaver KM, Yassaee M, Cameron HA. Short-term and long-term survival of new neurons in the rat dentate gyrus. J Comp Neurol. 2003;460:563–572. doi: 10.1002/cne.10675. [DOI] [PubMed] [Google Scholar]
- Deak T, Bordner KA, McElderry NK, Barnum CJ, Blandino P, Jr, Deak MM, Tammariello SP. Stress-induced increases in hypothalamic IL-1: a systematic analysis of multiple stressor paradigms. Brain Res Bull. 2005;64:541–56. doi: 10.1016/j.brainresbull.2004.11.003. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New Neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droste SK, Gesing A, Ulbricht S, Muller MB, Linthorst AC, Reul JM. Effects of long-term voluntary exercise on the mouse hypothalamic-pituitary-adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Dupret D, Revest JM, Koehl M, Ichas F, De Giorgi F, Costet P, Abrous DN, Piazza PV. Spatial relational memory requires hippocampal adult neurogenesis. PLoS One. 2008;3:e1959. doi: 10.1371/journal.pone.0001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehninger D, Kempermann G. Paradoxical effects of learning the Morris water maze on adult hippocampal neurogenesis in mice may be explained by a combination of stress and physical activity. Genes Brain Behav. 2006;5:29–39. doi: 10.1111/j.1601-183X.2005.00129.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci USA. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Haack AK, Galea LA. Task difficulty in the Morris water task influences the survival of new neurons in the dentate gyrus. Hippocampus. 2010;20:866–876. doi: 10.1002/hipo.20692. [DOI] [PubMed] [Google Scholar]
- Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11:397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Fray P, Kostarczyk E, Taylor S, Stacey P. Studies of instrumental behavior with sexual reinforcement in male rats (Rattus norvegicus): I. Control by brief visual stimuli paired with a receptive female. J Comp Psychol. 1987;101:395–406. [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death, and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Farioli-Vecchioli S, Saraulli D, Costanzi M, Pacioni S, Cina I, Aceti M, Micheli L, Bacci A, Cestari V, Tirone F. The timing of differentiation of adult hippocampal neurons is crucial for spatial memory. PLoS One. 2008;6:e246. doi: 10.1371/journal.pbio.0060246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragud A, Haro A, Sylvain A, Velazquez-Sanchez C, Hernandez-Rabaza V, Canales JJ. Enhanced habit-based learning and decreased neurogenesis in the adult hippocampus in a murine model of chronic social stress. Behav Brain Res. 2010;210:134–139. doi: 10.1016/j.bbr.2010.02.013. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Stevens J, Barson JR, Blakley GG, Patterson-Buckendahl P, Jacobs BL. Delayed suppression of hippocampal cell proliferation in rats following inescapable shocks. Brain Res. 2007;1130:48–53. doi: 10.1016/j.brainres.2006.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréchette M, Rennie K, Pappas BA. Developmental forebrain cholinergic lesion and environmental enrichment: behaviour, CA1 cytoarchitecture and neurogenesis. Brain Res. 2009;1252:172–82. doi: 10.1016/j.brainres.2008.11.082. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sandler A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004a;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004b;7:1123–1141. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- Garthe A, Behr J, Kempermann G. Adult-generated hippocampal neurons allow the flexible use of spatially precise learning strategies. PLoS One. 2009;4:e5464. doi: 10.1371/journal.pone.0005464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Goh EL, Sailor KA, Kitabatake Y, Ming GL, Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439:589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T, Trouche S, Massou I, Verret L, Zerwas M, Roullet P, Rampon C. Young hippocampal neurons are critical for recent and remote spatial memory in adult mice. Neuroscience. 2010;171:769–778. doi: 10.1016/j.neuroscience.2010.09.047. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippomcampal neurogenesis suppression. Mol Psychiatry. 2008;13:717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E. Hippocampal neurogenesis in adult Old World primates. Proc Natl Acad Sci USA. 1999a;96:5263–5267. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Cameron HA, Daniels DC, Woolley CS, McEwen BS. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LA, Fuchs E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J Neurosci. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Tanapat P. Lesion-induced proliferation of neuronal progenitors in the dentate gyrus of the adult rat. Neuroscience. 1997;80:427–436. doi: 10.1016/s0306-4522(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999b;3:186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;95:3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E, Woolley CS, McEwen BS. Short-term glucocorticoid manipulations affect neuronal morphology and survival in the adult dentate gyrus. Neuroscience. 1990;37:367–375. doi: 10.1016/0306-4522(90)90407-u. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Hastings NB, Gould E. Rapid extension of axons into the CA3 region by adult-generated granule cells. J Comp Neurol. 1999;413:146–154. doi: 10.1002/(sici)1096-9861(19991011)413:1<146::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Heine VM, Zareno J, Maslam S, Joels M, Lucassen PJ. Chronic stress in the adult dentate gyrus reduces cell proliferation near the vasculature and VEGF and Flk-1 protein expression. Eur J Neurosci. 2005;21:1304–1314. doi: 10.1111/j.1460-9568.2005.03951.x. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK. Role of the ventral subiculum in stress integration. Behav Brain Res. 2006;174:215–224. doi: 10.1016/j.bbr.2006.05.035. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schäfer MK, Young EA, Thompson R, Douglass J, Akil H, Watson SJ. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. J Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Morano MI, Akil H, Watson SJ. Contribution of the ventral subiculum to inhibitory regulation of the hypothalamo-pituitary-adrenocortical axis. Neuroendocrinol. 1995;7:475–482. doi: 10.1111/j.1365-2826.1995.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Hill MN, Kambo JS, Sun JC, Gorzalka BB, Galea LA. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviours. Eur J Neurosci. 2006;24:1845–1849. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- Höglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- Hundt AG, Premack D. Running as both a positive and negative reinforcer. Science. 1963;142:1087–1088. doi: 10.1126/science.142.3595.1087. [DOI] [PubMed] [Google Scholar]
- Hwang IK, Yoo KY, Li H, Choi JH, Kwon YG, Ahn Y, Lee IS, Won MH. Differences in doublecortin immunoreactivity and protein levels in the hippocampal dentate gyrus between adult and aged dogs. Neurochem Res. 2007;32:1604–1609. doi: 10.1007/s11064-007-9366-1. [DOI] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integration of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Jacobson L, Sapolsky R. The role of hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endo Reviews. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JD, O’Connor KA, Watkins LR, Maier SF. The role of IL-1beta in stress-induced sensitization of proinflammatory cytokine and corticosterone responses. Neuroscience. 2004;127:569–77. doi: 10.1016/j.neuroscience.2004.05.046. [DOI] [PubMed] [Google Scholar]
- Kambo JS, Galea LA. Activation levels of androgens influence risk assessment behaviour but do not influence stress-induced suppression in hippocampal cell proliferation in adult male rats. Behav Brain Res. 2006;175:263–270. doi: 10.1016/j.bbr.2006.08.032. [DOI] [PubMed] [Google Scholar]
- Kee N, Sivalingam S, Boonstra R, Wojtowicz JM. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippomcampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–143. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- Khawaja X, Xu J, Liang JJ, Barrett JE. Proteomic analysis of protein changes developing in rat hippocampus after chronic antidepressant treatment: implications for depressive disorders and future therapies. J Neurosci Res. 2004;75:451–460. doi: 10.1002/jnr.10869. [DOI] [PubMed] [Google Scholar]
- Kikusui T, Ichikawa S, Mori Y. Maternal deprivation by early weaning increases corticosterone and decreases hippocampal BDNF and neurogenesis in mice. Psychoneuroendocrinology. 2009;34:762–772. doi: 10.1016/j.psyneuen.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Saitoh Y, Takashima N, Murayama A, Niibori Y, Ageta H, Sekiguchi M, Sugiyama H, Inokuchi K. Adult neurogenesis modulates the hippocampus-dependent period of associative fear memory. Cell. 2009;139:814–827. doi: 10.1016/j.cell.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Klaus F, Hauser T, Slomianka L, Lipp HP, Amrein I. A reward increases running-wheel performance without changing cell proliferation, neuronal differentiation or cell death in the dentate gyrus of C57BL/6 mice. Brain Res. 2009;204:175–181. doi: 10.1016/j.bbr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, Volk B, Kempermann G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, Lee YS, Son H, Kaang BK. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl M, Meerlo P, Gonzales D, Rontal A, Turek FW, Abrous DN. Exercise-induced promotion of hippocampal cell proliferation requires β-endorphin. FASEB J. 2008;22:2253–2262. doi: 10.1096/fj.07-099101. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Morrison JH. Long-term gonadal hormone treatment and endogenous neurogenesis in the dentate gyrus of the adult female monkey. Exp Neurol. 2010;224:252–257. doi: 10.1016/j.expneurol.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani S, Yamauchi T, Teramoto T, Ogura H. Pharmacological evidence of cholinergic involvement in adult hippocampal neurogenesis in rats. Neuroscience. 2006;142:505–14. doi: 10.1016/j.neuroscience.2006.06.035. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Donovan MH, DeCarolis NA, Farnbauch LA, Malhotra S, Berton O, Nestler EJ, Krishnan V, Eisch AJ. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc Natl Acad Sci U S A. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagace DC, Fischer SJ, Eisch AJ. Gender and endogenous levels of estradiol do not influence adult hippocampal neurogenesis in mice. Hippocampus. 2007;17:175–180. doi: 10.1002/hipo.20265. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Kim SJ, Kim SW, Choi SH, Shin YC, Park SH, Moon BH, Cho E, Lee MS, Choi SH, Chun BG, Shin KH. Chronic mild stress decreases survival, but not proliferation, of new-born cells in adult rat hippocampus. Exp Mol Med. 2006;38:44–54. doi: 10.1038/emm.2006.6. [DOI] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–40. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Kozorovitskiy Y, Gross CG, Gould E. Diminished adult neurogenesis in the marmoset brain precedes old age. Proc Natl Acad Sci USA. 2007;104:17169–17173. doi: 10.1073/pnas.0708228104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neurogenesis in the hippocampus despite an initial elevation in stress hormones. PLoS One. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd SA, Balest ZR, Corotto FS, Smeyne RJ. Cocaine selectively increases proliferation in the adult murine hippocampus. Neurosci Lett. 2010;485:112–116. doi: 10.1016/j.neulet.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PE, Bolwig TG, Wörtwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Namba T, Suzuki E, Yuasa S, Kohsaka S, Uchino S. NMDA receptor antagonist memantine promotes cell proliferation and production of mature granule neurons in the adult hippocampus. Neurosci Res. 2009;63:259–66. doi: 10.1016/j.neures.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Makatsori A, Duncko R, Schwendt M, Moncek F, Johansson BB, Jezova D. Voluntary wheel running modulates glutamate receptor subunit gene expression and stress hormone release in Lewis rats. Psychoneuroendocrinology. 2003;28:702–714. doi: 10.1016/s0306-4530(02)00062-8. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Duman RS. Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology. 2003;28:1562–1571. doi: 10.1038/sj.npp.1300234. [DOI] [PubMed] [Google Scholar]
- Meshi D, Drew MR, Saxe M, Ansorge MS, David D, Santarelli L, Malapani C, Moore H, Hen R. Hippocampal neurogenesis is not required for behavioral effects of environmental enrichment. Nat Neurosci. 2006;9:729–31. doi: 10.1038/nn1696. [DOI] [PubMed] [Google Scholar]
- Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–846. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sundlass K, Parker KJ, Schatzberg AF, Lyons DM. Social stress-related behavior affects hippocampal cell proliferation in mice. Physiol Behav. 2006;89:123–127. doi: 10.1016/j.physbeh.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Johansson BB, Jezova D. Effect of environmental enrichment on stress related systems in rats. J Neuroendocrinol. 2004;16:423–431. doi: 10.1111/j.1365-2826.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- Morton J, Leuner B, Gould E. The effects of oxytocin on the proliferation of neuronal precursors in the adult hippocampus. Society for Neuroscience Abstr. 2009 [Google Scholar]
- Moser E, Moser MB, Andersen P. Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci. 1993;13:3916–3925. doi: 10.1523/JNEUROSCI.13-09-03916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen CA, Mayer JL, de Kloet ER, Joels M, Lucassen PJ. Brief treatment with the glucocorticoid receptor antagonist mifepristone normalizes the reduction in neurogenesis after chronic stress. Eur J Neurosci. 2007;26:3395–3401. doi: 10.1111/j.1460-9568.2007.05972.x. [DOI] [PubMed] [Google Scholar]
- O’Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Perera TD, Coplan JD, Lisanby SH, Lipira CM, Arif M, Carpio C, Spitzer G, Santarelli L, Scharf B, Hen R, Rosoklija G, Sackelm HA, Dwork AJ. Antidepressant-induced neurogenesis in the hippocampus of adult nonhuman primates. J Neurosci. 2007;27:4894–4901. doi: 10.1523/JNEUROSCI.0237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PF, McEwen BS. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci. 2003;17:879–886. doi: 10.1046/j.1460-9568.2003.02513.x. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, LeFevour A, Morhardt D, Curley J, Mizumatsu S, VandenBerg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: Microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Korn MJ, Shan Z, Obenaus A. Dendritic growth cones and recurrent basal dendrites are typical features of newly generated dentate granule cells in the adult hippocampus. Brain Res. 2004;1000:195–199. doi: 10.1016/j.brainres.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Rola R, Raber J, Rizk A, Otsuka S, VandenBerg SR, Morhardt DR, Fike JR. Radiation-induced impairment of hippocampal neurogenesis is associated with cognitive deficits in young mice. Exp Neurol. 2004;188:316–330. doi: 10.1016/j.expneurol.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur J Neurosci. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sairanen M, Lucas G, Ernfors P, Castren M, Castren E. Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation, and survival in the adult dentate gyrus. J Neurosci. 2005;25:1089–1094. doi: 10.1523/JNEUROSCI.3741-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger AR, Cowan WM, Gottlieb DI. An autoradiographic study of the time of origin and the pattern of granule cell migration in the dentate gyrus of the rat. J Comp Neurol. 1975;159:149–175. doi: 10.1002/cne.901590202. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Aguilera G, Binnekade R, Tilders FJ. Single administration of interleukin-1 increased corticotropin releasing hormone and corticotropin releasing hormone-receptor mRNA in the hypothalamic paraventricular nucleus which paralleled long-lasting (weeks) sensitization to emotional stressors. Neuroscience. 2003;116:275–83. doi: 10.1016/s0306-4522(02)00555-9. [DOI] [PubMed] [Google Scholar]
- Seki T, Arai Y. Age-related production of new granule cells in the adult dentate gyrus. Neuroreport. 1995;6:2479–2482. doi: 10.1097/00001756-199512150-00010. [DOI] [PubMed] [Google Scholar]
- Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A. Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci. 2001;21:7153–7160. doi: 10.1523/JNEUROSCI.21-18-07153.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy T, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12:578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M, Czeh B, Fuchs E. Age-dependent susceptibility of adult hippocampal cell proliferation to chronic psychosocial stress. Brain Res. 2005;1049:244–248. doi: 10.1016/j.brainres.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Sloviter RS, Valiquette G, Abrams GM, Ronk EC, Solias AL, Paul LA, Neubort S. Selective loss of hippocampal granule cells in the mature rat brain after adrenalectomy. Science. 1989;243:535–538. doi: 10.1126/science.2911756. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Brewer M, Glover L, Sanzone K, Cameron H. Adult hippocampal neurogenesis regulates the response to stress. Soc. Neurosci. Abstr. 2010 [Google Scholar]
- Snyder JS, Chloe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009a;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Glover LR, Sanzone KM, Kamhi JF, Cameron HA. The effects of exercise and stress on the survival and maturation of adult-generated granule cells. Hippocampus. 2009b;19:898–906. doi: 10.1002/hipo.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Hong NS, McDonald RJ, Wojtowicz JM. A role for adult neurogenesis in spatial long-term memory. Neuroscience. 2005;130:843–52. doi: 10.1016/j.neuroscience.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Kee N, Wojtowicz JM. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol. 2001;85:2423–31. doi: 10.1152/jn.2001.85.6.2423. [DOI] [PubMed] [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia. 2004;46:41–52. doi: 10.1002/glia.10337. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533. doi: 10.1038/nn1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Zhu Y, Hata T, Shimizu-Okabe C, Suzuki K, Nakahara D. Intracranial self-stimulation enhances neurogenesis in hippocampus of adult mice and rats. Neuroscience. 2009;158:402–411. doi: 10.1016/j.neuroscience.2008.10.048. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Stall N, Dar DE, Zangen A. Knockdown of brain-derived neurotrophic factor in specific brain sites precipitates behaviors associated with depression and reduces neurogenesis. Mol Psychiatry. 2010;15:80–92. doi: 10.1038/mp.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Rydel TA, Galea LA, Gould E. Exposure to fox odor inhibits cell proliferation in the hippocampus of adult rats via an adrenal hormone-dependent mechanism. J Comp Neurol. 2001;437:496–504. doi: 10.1002/cne.1297. [DOI] [PubMed] [Google Scholar]
- Thomas RM, Hotsenpiller G, Peterson DA. Acute psychosocial stress reduces cell survival in adult hippocampal neurogenesis without altering proliferation. J Neurosci. 2007;27:2734–2743. doi: 10.1523/JNEUROSCI.3849-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas RM, Urban JH, Peterson DA. Acute exposure to predator odor elicits a robust increase in corticosterone and a decrease in activity without altering proliferation in the adult rat hippocampus. Exp Neurol. 2006;201:308–315. doi: 10.1016/j.expneurol.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Tenk CM, Wilson H, Zhang Q, Pitchers KK, Coolen LM. Sexual reward in male rats: effects of sexual experience on conditioned place preferences associated with ejaculation and intromissions. Horm Behav. 2009;55:93–97. doi: 10.1016/j.yhbeh.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Harst JE, Fermont PCJ, Bilstra AE, Spruijt BM. Access to enriched housing is rewarding to rats as reflected by their anticipatory behaviour. Animal Behavior. 2003;66:493–504. [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Mahati K, Bhagya V, Raju TR, Rao B.S. Shankaranarayana. Enriched environment restores hippocampal cell proliferation and ameliorates cognitive deficits in chronically stressed rats. J Neurosci Res. 2009a;87:831–843. doi: 10.1002/jnr.21907. [DOI] [PubMed] [Google Scholar]
- Veena J, Srikumar BN, Raju TR, Rao B.S. Shankaranarayana. Exposure to enriched environment restores the survival and differentiation of new born cells in the hippocampus and ameliorates depressive symptoms in chronically stressed rats. Neurosci Lett. 2009b;455:178–182. doi: 10.1016/j.neulet.2009.03.059. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Madsen TM, Duman RS. Electroconvulsive seizure restores neurogenesis and hippocampus-dependent fear memory after disruption by irradiation. Eur J Neurosci. 2008;27:1485–1493. doi: 10.1111/j.1460-9568.2008.06118.x. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Winner B, Desplats P, Hagl C, Klucken J, Aigner R, Ploetz S, Laemke J, Karl A, Aigner L, Masliah E, Buerger E, Winkler J. Dopamine receptor activation promotes adult neurogenesis in an acute Parkinson model. Exp Neurol. 2009;219:543–552. doi: 10.1016/j.expneurol.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocur G, Wojtowicz JM, Sekeres M, Snyder JS, Wang S. Inhibition of neurogenesis interferes with hippocampus-dependent memory function. Hippocampus. 2006;16:296–304. doi: 10.1002/hipo.20163. [DOI] [PubMed] [Google Scholar]
- Wong EY, Herbert J. Raised circulating corticosterone inhibits neuronal differentiation of progenitor cells in the adult hippocampus. Neuroscience. 2006;137:83–92. doi: 10.1016/j.neuroscience.2005.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, Gould E, Sakai RR, Spencer RL, McEwen BS. Effects of aldosterone or RU28362 treatment on adrenalectomy-induced cell death in the dentate gyrus of the adult rat. Brain Res. 1991;554:312–315. doi: 10.1016/0006-8993(91)90207-c. [DOI] [PubMed] [Google Scholar]
- Xu Y, Ku B, Cui L, Li X, Barish PA, Foster TC, Ogle WO. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007;1162:9–18. doi: 10.1016/j.brainres.2007.05.071. [DOI] [PubMed] [Google Scholar]
- Yap JJ, Takase LF, Kochman LJ, Fornal CA, Miczek KA, Jacobs BL. Repeated brief social defeat episodes in mice: effects on cell proliferation in the dentate gyrus. Behav Brain Res. 2006;172:344–350. doi: 10.1016/j.bbr.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2009;34:1039–1046. doi: 10.1007/s11064-008-9870-y. [DOI] [PubMed] [Google Scholar]
- Ying Z, Roy RR, Edgerton VR, Gomez-Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhao C, Teng EM, Summers RG, Jr, Ming GL, Gage FH. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci. 2006;26:3–11. doi: 10.1523/JNEUROSCI.3648-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuena AR, Mairesse J, Casolini P, Cinque C, Alemá GS, Morley-Flethcer S, Chiodi V, Spagnoli LG, Gradini R, Catalani A, Nicoletti F, Maccari S. Prenatal restraint stress generates two distinct behavioral and neurochemical profiles in male and female rats. PLoS One. 2008;3:e2170. doi: 10.1371/journal.pone.0002170. [DOI] [PMC free article] [PubMed] [Google Scholar]