Fig 4.
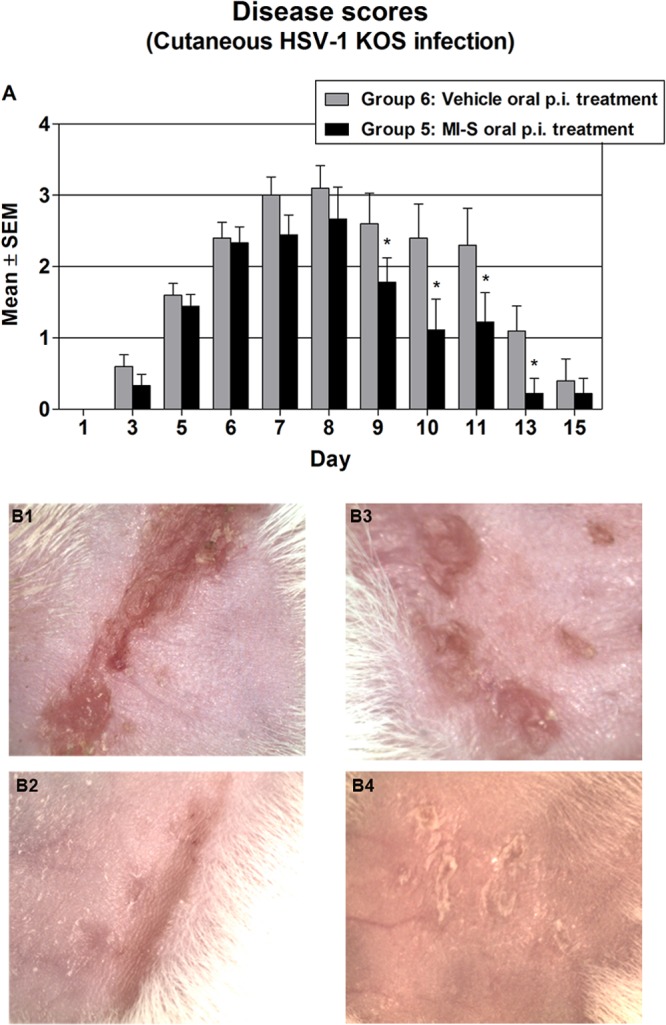
Effect of MI-S postinfection oral treatment on HSV-1 cutaneous disease. (A) Groups of 10 mice were treated after infection by oral gavage (100 μl), as follows: group 6, vehicle treatment, whereby mice were treated with PBS twice a day for 7 days; group 5, MI-S treatment, whereby MI-S solution was administered twice a day (2 mg/day) for 7 days. The severity of disease was scored from day 1 to day 15 as described in Materials and Methods. Values are expressed as means ± SEM from 10 mice in each group. Groups were compared by two-way ANOVA followed by the Bonferroni posttest; *, P < 0.05. (B) Representative examples of cutaneous infection at day 8. (B1) Zosteriform lesion, group 6, score 4; (B2) zosteriform healing lesion, group 5, score 3; (B3) spreading ulceration, group 6, score 3; (B4) healing ulcers, group 5, score 2.
