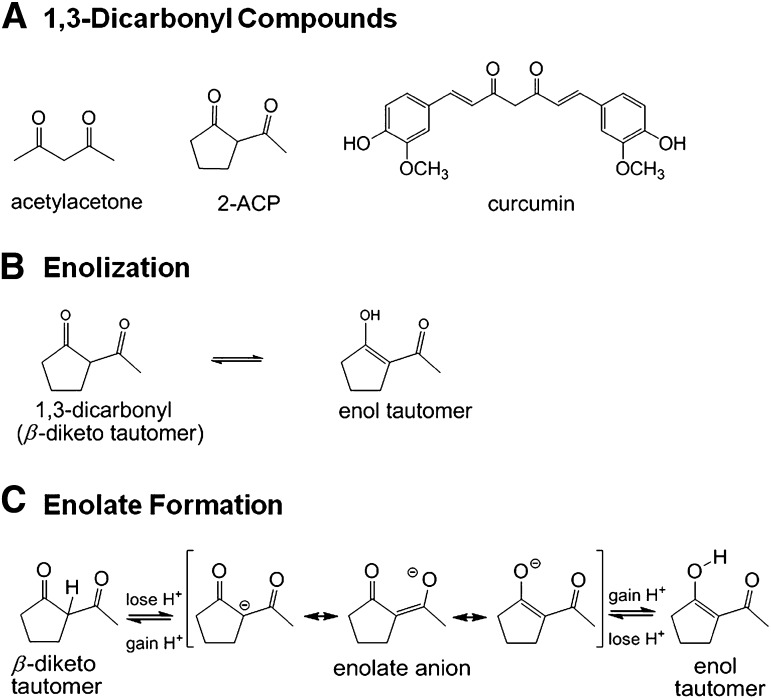
Fig. 1.
Line structures and ionization of 1,3-dicarbonyl compounds. (A) Line structures for acetylacetone, 2-acetylcyclopentanone (2-AC), and curcumin. (B) Schematic diagram illustrating the existence of 2-ACP as equilibrating keto-enol tautomers. (C) Schematic diagram showing that loss of a proton from either the central (“α”) carbon in the diketo tautomer of 2-ACP or the hydroxyl group of the enol isomer yields the same resonance-stabilized enolate anion.