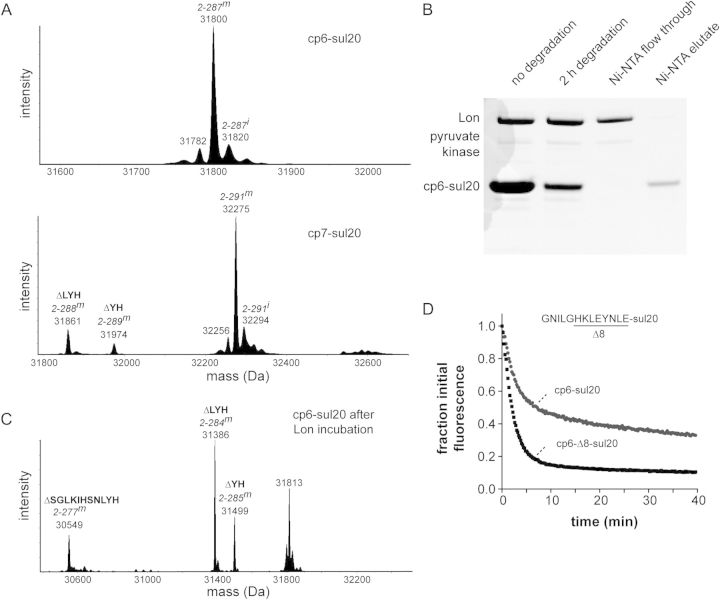
Fig. 2.
(A) Deconvoluted electrospray ionization mass spectra of the purified cp6-sul20 (top) and cp7-sul20 (bottom) proteins. Full-length cp6-sul20 with a mature chromophore but lacking the N-terminal methionine (residue 2–287m) has an expected mass of 31 800.8 Da. Prior to chromophore maturation (2–287i), the expected mass is 20 Da higher. Full-length cp7-sul20 with a mature chromophore but lacking the N-terminal methionine (residues 2–291m) has an expected mass of 32 274.3 Da. Small amounts of proteins missing two (ΔYH) or three residues (ΔLYH) from the C-terminal end of the sul20 degron were observed in the cp7-sul20 but not the cp6-sul20 preparation. (B) The cp6-sul20 protein (50 µM), which contains an N-terminal His6 tag, was incubated with Lon6 (1 µM) for 2 h at 37°C and then purified by Ni++-affinity chromatography. (C) Electrospray ionization mass spectrometry of cp6-sul20 after 2 h of Lon degradation and Ni++-affinity purification revealed substantial tail clipping that removes 2 (ΔYH), 3 (ΔLYH) or 11 (ΔSGLKIHSNLYH) C-terminal residues from the sul20 degron. (D) Degradation of cp6-sul20 or cp6-Δ8-sul 20 (0.5 µM each) by Lon6 (2 µM) at 37°C. Degradation was monitored by loss of 511 nm fluorescence after excitation at 467 nm. Fluorescence was normalized to an initial value of 1.