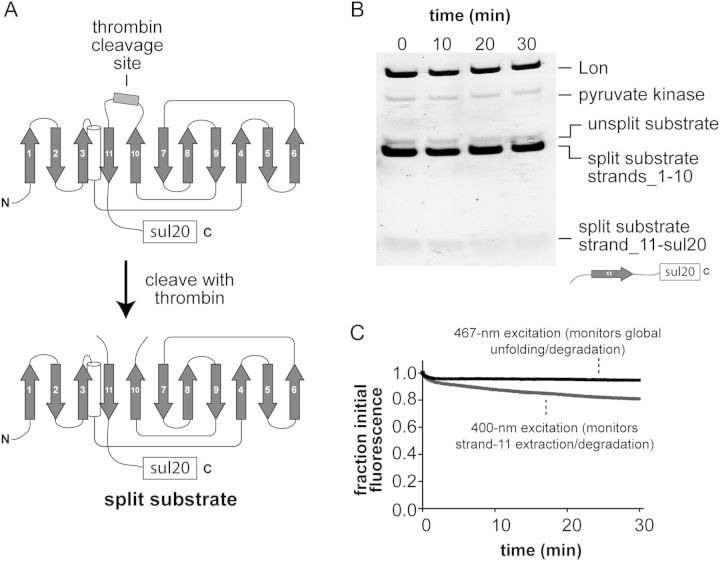
Fig. 3.
(A) Cartoon showing the thrombin-cleavage site inserted between β-strands 10 and 11 of GFP-sul20 and the generation of the split substrate by thrombin cleavage. (B) The thrombin-split GFP-sul20 protein (5 µM) was incubated with Lon6 (0.3 µM) and the reaction was monitored by SDS-PAGE, followed by staining with Coomassie Blue. (C) The thrombin-split GFP-sul20 protein (0.5 µM) was incubated with Lon6 (2 µM) and the reaction was monitored by changes in fluorescence at 511 nm after excitation at 400 nm (extraction/degradation of β-strand 11) or 467 nm (global unfolding/degradation).